2474
Sex-specific grey matter atrophy and brain-behavior relationships in CADASIL1Beijing Chaoyang Hospital, Capital Medical University, Beijing, China, 2Peking University First Hospital, Beijing, China, 3MR Scientific Marketing, Siemens Healthineers, Beijing, China, 4USC Mark & Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 5State Key Laboratory of Brain and Cognitive Science, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China
Synopsis
Keywords: Blood vessels, Brain, CADASIL
The potential neurobiological substrates for sex differences in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) are largely unexplored. In this study, we explored the sex-specific neuroanatomical mechanisms of CADASIL. Greater grey matter atrophy was found in the frontotemporal cortex in male CADASIL. Working memory was associated with volumes in the bilateral orbitofrontal cortex and left entorhinal cortex among female CADASIL. The current findings indicate that sex affects the pathogenesis of CADASIL, ranging from differences in neuroanatomy to those in behavioral performance.Introduction
Brain damage caused by small vessel disease (SVD) differs between males and females[1]. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a pure SVD characterized by degeneration or loss of vascular smooth muscle cells (VSMCs) in small arteries and arterioles and deposits of granular osmiophilic material (GOM) in vessel walls [2]. It has been reported that patients with CADASIL exhibit sex-specific protection against disease progression [3][4], and the potential neurobiological substrates for sex differences in CADASIL are largely unexplored. Furthermore, few MRI studies have investigated sex differences in brain morphological changes in CADASIL patients. However, disease-specific sex differences in anatomical changes in CADASIL cannot be determined from existing observations due to a lack of knowledge about sex differences in age-matched healthy brains. Voxel-based morphological (VBM) assessment of brain tissue is required to detect subtle sex-specific changes in volume by high-resolution MRI. This study aimed to examine the sex-specific neuroanatomical mechanisms of CADASIL, a hereditary SVD, and to reveal the underlying brain-behavior associations in male and female CADASIL patients.Methods
Sixty-three patients (31 females, 43.74 ± 11.43 years of age, and 32 males, 42.03 ± 8.92 years of age) with CADASIL confirmed via genetic analysis or skin biopsy were recruited. The control group consisted of 55 healthy volunteers (28 females, 43.12 ± 11.52 years of age, and 27 males, 40.52 ± 11.01 years of age) matched for age and sex ratio. This study was approved by the Institutional Review Board and Ethics Committee of Peking University First Hospital, and written informed consent was obtained from each participant. All patients underwent a modified Rankin Scale (mRS) score assessment to evaluate their degree of disability/dependence and a comprehensive neuropsychological assessment or Mini-Mental State Examination (MMSE) to evaluate their cognitive function. MRI data were acquired using a MEGNETOM 7.0T scanner (Siemens Healthcare, Germany) with a 32-channel head coil. High-resolution structural images were acquired using 3D T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE). The scan parameters were as follows: TR = 3000 ms, TE = 3.76 ms, flip angle = 8°, slice thickness = 0.7 mm, matrix = 320 × 320, FOV = 224 × 224 mm2, resolution = iso-0.7mm. T2-weighted fluid-attenuated inversion recovery images were acquired using the following parameters: TR = 14,000 ms, TE = 94 ms, flip angle = 120°, slice thickness = 3 mm, matrix = 95 × 114, and FOV = 220 × 199 mm2.VBM and deformation-based morphometry (DBM) analyses were performed. W-score maps were calculated from volumes and deformations of brain tissue, controlling normal effects of age and sex in healthy controls. Regression analyses were performed to investigate sex-specific relationships between these quantitative alterations in brain structure and behavioral performance in CADASIL patients.Results and Discussion
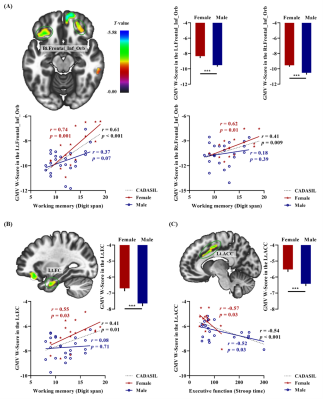
Sex-specific anatomical alterations in CADASIL were confirmed in this study by reporting GM volume alterations in conjunction with those of DBM, which indicated that these sex differences in GM alterations in CADASIL were robust. Specifically, compared with female patients with CADASIL, males exhibited significantly greater GM atrophy in the bilateral inferior orbitofrontal cortex (medial and lateral part), left ACC, left EC and right temporooccipital cortex including the middle temporal gyrus and the primary visual area hOc1. However, no more severe GM atrophy was found in women with CADASIL than in men. We also explored the neuroanatomical substrates underlying the significantly higher behavioral performance on assessments of working memory and executive function in female patients with CADASIL. As shown in Figure 1, a significant correlation was detected between working memory performance and GMV W-scores in the bilateral inferior orbitofrontal cortex (r = 0.74, p < 0.001 for the left side; r = 0.62, p = 0.01 for the right side) (see Figure 1A) and in left EC (r = 0.55, p = 0.03) (see Figure 1B) specific to female patients with CADASIL. Additionally, GMV W-scores in the left ACC were significantly associated with executive function in both female (r = -0.57, p = 0.03) and male patients with CADASIL (r = -0.52, p = 0.03) (see Figure 1C).Conclusion
The current findings suggest sex effects in CADASIL pathogenesis, ranging from differences in neuroanatomy to differences in behavioral performance. These findings may contribute to the development of more effective sex-specific therapeutic interventions for CADASIL and SVD.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81961128030, 82025018, 62076169, 82001804, and 31730039), Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20180602), Natural Science Foundation of Beijing Municipality (7191003), Ministry of Science and Technology of China (2019YFA0707103), Beijing Hospitals Authority Youth Program (QML20200304), and Chinese Academy of Sciences (XDB32010300).References
[1] Jiménez-Sánchez L, Hamilton OKL, Clancy U, Backhouse EV, Stewart CR, Stringer MS, Doubal FN and Wardlaw JM. Sex differences in cerebral small vessel disease: A systematic review and meta-analysis. Front Neurol 2021; 12:756887.
[2] Kalimo H, Ruchoux MM, Viitanen M, Kalaria RN. CADASIL: a common form of hereditary arteriopathy causing brain infarcts and dementia. Brain Pathol 2002; 12:371–384.
[3] Dichgans M, Mayer M, Uttner I, Bruning R, Muller-Hocker J, Rungger G, Ebke M, Klockgether T, Gasser T. The phenotypic spectrum of CADASIL: Clinical findings in 102 cases. Ann Neurol 1998; 44(5):731–739.
[4] Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain 2004; 127:2533–2539.
Figures