2440
Free-Breathing, Gadoxetic Acid Enhanced, 3D T1w Phase Sensitive Inversion Recovery Hepatobiliary MRI Optimized for 3.0 Tesla1Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Global MR Applications and Workflow, GE Healthcare, Waukesha, WI, United States, 4Global MR Applications and Workflow, GE Healthcare, Atlanta, GA, United States, 5Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 6Department of Medicine, University of Wisconsin-Madison, Madison, WI, United States, 7Department of Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Keywords: Liver, Image Reconstruction
Gadoxetic acid (GA)-enhanced hepatobiliary phase (HBP) T1-weighted (T1w) MRI is well established for the detection of focal liver lesions. Previously, we proposed a novel imaging method to improve T1 contrast in GA-enhanced HBP T1w images for the purpose of improved detection and characterization of liver lesions. The proposed method provides volumetric, high-resolution phase-sensitive inversion recovery (PSIR) T1w images of the liver from a free-breathing image acquisition. This study focuses on the impact of flip angle and spoiling on image quality. Our findings indicate 6-8 degrees flip angle in combination with gradient spoiling provides optimal image quality and T1 contrast.Introduction
Gadoxetic acid (“GA”, Eovist / Primovist, Bayer Healthcare)-enhanced, hepatobiliary phase (HBP) T1-weighted (T1w) MRI has become an essential tool for detection of liver lesions1,2. However, GA-enhanced T1w MRI alone cannot delineate the differences between benign and malignant lesions. While T2w and diffusion weighted abdominal MRI contribute to the characterization of focal liver lesions, they often cannot resolve smaller (<1cm) lesions due to limitations on spatial resolution3-6. As a result, high resolution GA-enhanced HBP T1w acquisitions are often the only sequence to detect small lesions, but on their own, are unable to characterize lesions as benign or malignant.In a previous study, we proposed a novel method to improve T1 contrast of GA-enhanced T1w-MRI through combination of inversion recovery (IR) and phase sensitive (PS) image reconstruction7. Furthermore, this method utilized stack-of-stars imaging for free-breathing image acquisition and chemical shift encoded (CSE) imaging for improved water/fat separation8,9. Finally, a model-based compressed sensing image reconstruction algorithm was developed as a part of this study to reconstruct multi-T1w images7,10.
Previous studies have investigated the optimal imaging parameters such as flip angle for T1w 3D Spoiled Gradient Echo MRI at 1.5 T field strength, however use of magnetization preparation and CSE-MRI techniques requires the re-evaluation of imaging parameters for the proposed method6. Therefore, the purpose of this work is to investigate the impact of flip angle and spoiling method on the resulting image quality of PSIR T1w HBP GA-enhanced MRI.
Theory
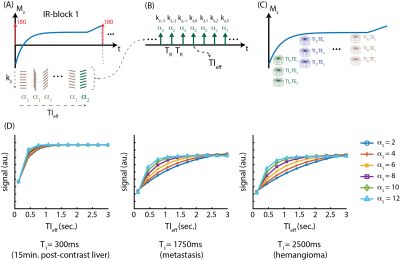
MRI Acquisition and Reconstruction: An adiabatic inversion pulse is used to invert the longitudinal magnetization, followed by the acquisition of a series of radial stacks of single echo kz-readouts. This acquisition scheme is repeated for three-echoes. Last TI frame is acquired with a small flip angle to minimize T1 contrast on the phase reference11. The proposed image acquisition scheme is illustrated in figure 1. Raw k-space data are later synchronized according to the echo time (TE) and effective inversion time (TIeff), where T1 contrast of the resulting images are determined by TIeff14,18.Apparent T1 Relaxation: The application of an inversion pulse, followed by a series of imaging RF pulses, impacts the trajectory of the longitudinal signal recovery, resulting in an “apparent” T1 relaxation12,13. Figure 1d shows Bloch equation simulations of the apparent T1 relaxation for different T1 species (e.g., post-contrast liver, metastasis, and hemangioma) and different flip angles. As illustrated here, apparent T1 relaxation strongly depends on the flip angle, and flip angles as small as 8o may saturate the apparent T1 contrast, where images reconstructed at longer TIeff (e.g. TIeff > 1.5s) have negligible contrast changes.
Methods
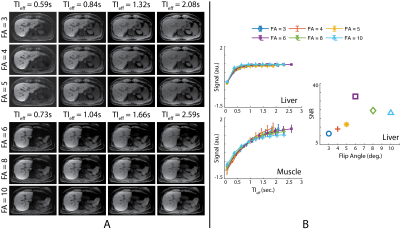
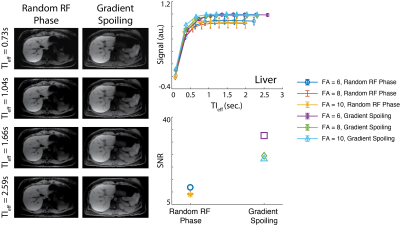
Optimal Flip Angle: Beginning at approximately 20 minutes after the contrast injection, multiple free-breathing acquisitions were performed with flip angles, varying from 3-10o in an increasing order to determine optimal flip angle to maximize the contrast between different tissues (e.g., liver, muscle, and spleen) and SNR, while minimizing apparent T1 contrast saturation in images with longer TIeff.Spoiling Method: Random RF phase spoiling and gradient spoiling in x- and z-dimensions were utilized to test the robustness of water/fat separation and image quality14.
Healthy Volunteer Study: The proposed method was tested in 8 healthy volunteers on a 3.0T MRI system (Discovery MR 750, GE Healthcare, Waukesha, WI) using a 32-channel phased array torso coil. PSIR images were acquired starting at 20 minutes after injection of 0.025 mmol/kg GA. Imaging parameters were: FOV=400x400x130mm3, resolution=1x1x2mm3, TR/TE1/$$$\Delta$$$TE=7/2.2/0.8ms, Bandwidth=166.67kHz. Total acquisition time was 10 minutes. Partial Fourier fraction of 70% in kz-dimension was used to shorten the scan time.
Results
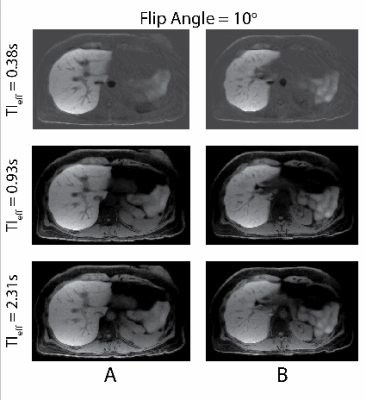
Optimal Flip Angle: Figure 2A shows the images acquired with flip angles ranging from 3 to 10o and reconstructed at various TIeff. Fig. 2B shows T1 contrast evolution over time and SNR performance obtained at various flip angles. T1 recovery was observed to saturate faster with larger flip angles, causing subtle contrast changes in images reconstructed at longer TIeff. Images acquired with larger flip angles outperformed the rest in SNR. Distinct T1 recovery behavior was captured in tissues with distinct T1 relaxations (e.g., liver and muscle). Figure 3 shows 3D volume reconstructed with the proposed method.Spoiling Method: Figure 4 shows images acquired with different spoiling methods. Water/fat separation did not improve with random RF phase spoiling, however SNR performance improved slightly with gradient spoiling.
Discussion
In this work, we presented an image parameter optimization study for a novel free-breathing, GA-enhanced, T1w MRI method. Results of the healthy volunteer study showed that small flip angles such as 3o can capture the T1 recovery behavior more accurately. However, these images suffer from low SNR. Larger flip angles can saturate the apparent T1 relaxation more quickly, resulting in negligible contrast changes in images reconstructed at longer TIeff. Random RF phase spoiling did not improve the water/fat separation, however resulted in slightly lower SNR due to use of a modified golden angle sampling scheme. This result agrees with the findings of Roeloffs, et al., where random RF phase spoiling in combination with golden angle sampling resulted in lower SNR performance14. Therefore, we conclude that 6-8o flip angles and gradient spoiling are optimal for the hepatobiliary phase GA-enhanced PSIR T1w imaging of the liver at 3.0T.Acknowledgements
We wish to acknowledge investigator-initiated research support from Bayer, UW Institute for Clinical and Translational Research, and the Clinical and Translational Science Award of the NCATS/NIH (UL1TR002373). Further, we wish to acknowledge GE Healthcare who provides research support to the University of Wisconsin. Finally, Dr. Reeder is a Fred Lee Sr. Endowed Chair of Radiology, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.References
[1] M. R. Bashir, D. B. Husarik, T. J. Ziemlewicz, R. T. Gupta, D. T. Boll, and E. M. Merkle, “Liver MRI in the hepatocyte phase with gadolinium-EOB-DTPA: Does increasing the flip angle improve conspicuity and detection rate of hypointense lesions?,” J. Magn. Reson. Imaging, vol. 35, no. 3, pp. 611–616, Mar. 2012, doi: 10.1002/jmri.22850.
[2] V. Vilgrain, M. Esvan, M. Ronot, A. Caumont-Prim, C. Aubé, and G. Chatellier, “A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases,” Eur Radiol, vol. 26, no. 12, pp. 4595–4615, Dec. 2016, doi: 10.1007/s00330-016-4250-5.
[3] P. Bannas, U. Motosugi, D. Hernando, M. S. Rahimi, J. H. Holmes, and S. B. Reeder, “Combined gadoxetic acid and gadofosveset enhanced liver MRI: A feasibility and parameter optimization study: Combined Gadoxetic Acid and Gadofosveset Liver MRI,” Magn. Reson. Med., vol. 75, no. 1, pp. 318–328, Jan. 2016, doi: 10.1002/mrm.25554.
[4] U. Motosugi, P. Bannas, D. Hernando, M. Salmani Rahimi, J. H. Holmes, and S. B. Reeder, “Intraindividual Crossover Comparison of Gadoxetic Acid Dose for Liver MRI in Normal Volunteers,” MRMS, vol. 15, no. 1, pp. 60–72, 2016, doi: 10.2463/mrms.2015-0005.
[5] A. Frydrychowicz, S. K. Nagle, S. L. D’Souza, K. K. Vigen, and S. B. Reeder, “Optimized high-resolution contrast-enhanced hepatobiliary imaging at 3 tesla: A cross-over comparison of gadobenate dimeglumine and gadoxetic acid,” Journal of Magnetic Resonance Imaging, vol. 34, no. 3, pp. 585–594, Sep. 2011, doi: 10.1002/jmri.22713.
[6] S. K. Nagle et al., “High resolution navigated three-dimensional T1-weighted hepatobiliary MRI using gadoxetic acid optimized for 1.5 tesla,” J. Magn. Reson. Imaging, vol. 36, no. 4, pp. 890–899, Oct. 2012, doi: 10.1002/jmri.23713.
[7] Y. Muslu, T. A. Cashen, S. Mandava, and S. B. Reeder, “Free-Breathing, 3D Phase Sensitive Inversion Recovery T1-Weighted MRI of the Liver,” In Proceedings of 31st Annual Meeting of ISMRM, London, England, UK, 2022.
[8] L. Feng et al., “Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI: iGRASP: Iterative Golden-Angle RAdial Sparse Parallel MRI,” Magn. Reson. Med., vol. 72, no. 3, pp. 707–717, Sep. 2014, doi: 10.1002/mrm.24980.
[9] Reeder, S.B., Pineda, A.R., Wen, Z., Shimakawa, A., Yu, H., Brittain, J.H., Gold, G.E., Beaulieu, C.H. and Pelc, N.J. (2005), Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): Application with fast spin-echo imaging. Magn. Reson. Med., 54: 636-644. Doi: 10.1002/mrm.20624
[10] J. I. Tamir et al., “T2 shuffling: Sharp, multicontrast, volumetric fast spin-echo imaging,” Magnetic Resonance in Medicine, vol. 77, no. 1, pp. 180–195, Jan. 2017, doi: 10.1002/mrm.26102.
[11] P. Kellman, A. E. Arai, E. R. McVeigh, and A. H. Aletras, “Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement,” Magn. Reson. Med., vol. 47, no. 2, pp. 372–383, Feb. 2002, doi: 10.1002/mrm.10051.
[12] R Deichmann, A Haase, “Quantification of T1 values by SNAPSHOT-FLASH NMR imaging,” Journal of Magnetic Resonance (1969), Volume 96, Issue 3, 1992, Pages 608-612, doi: 10.1016/0022-2364(92)90347-A.
[13] D. R. Messroghli, A. Radjenovic, S. Kozerke, D. M. Higgins, M. U. Sivananthan, and J. P. Ridgway, “Modified Look-Locker inversion recovery (MOLLI) for high-resolutionT1 mapping of the heart,” Magn. Reson. Med., vol. 52, no. 1, pp. 141–146, Jul. 2004, doi: 10.1002/mrm.20110.
[14] Roeloffs V, Voit D, Frahm J. Spoiling without additional gradients: Radial FLASH MRI with randomized radiofrequency phases. Magn Reson Med. 2016 May;75(5):2094-9. doi: 10.1002/mrm.25809.
Figures