2437
Prediction for aggressiveness and recurrence of hepatocellular carcinoma using gadoxetic acid-enhanced magnetic resonance imaging1Nantong Third People's Hospital, Nantong, China, 2Philips Healthcare, Nantong, China
Synopsis
Keywords: Cancer, MR Value
This study explores the predictive value of gadoxetic acid-enhanced magnetic resonance imaging (MRI) features on the pathologic grade, microvascular invasion (MVI), and cytokeratin-19 (CK19) expression in hepatocellular carcinomas (HCC), and evaluates their association with postoperative recurrence of HCC. The main method is to investigate whether there are independent predictors of the above aggressive indicators in the gadoxetic acid-enhanced MRI features and clinical parameters. Our study shows that peritumoral enhancement, peritumoral hypointensity and rim enhancement could independently predict poor pathological grade, MVI and CK19, respectively. Consequently, these results may help HCC patients develop more appropriate treatment options.Introduction
Hepatocellular carcinoma is ranked as the fourth leading cause of cancer-related deaths worldwide, which is related to its aggressiveness and high recurrence rate. It is known that high pathologic grade in HCCs is closely related to aggressive tumor behaviour and recurrence after surgery. Likewise, it is well known that MVI is associated with early HCC recurrence. Curative treatments vary depending on whether the patients have MVI. CK19 was found to be a crucial biological marker of the proliferative subtype, a high-risk subgroup which is always accompanied by high rates of recurrence and metastases in patients with HCC. Furthermore, it has been demonstrated that CK19-positive HCC cells were associated with invasion, angiogenesis and epithelial-mesenchymal transition (EMT). Accordingly, it is essential to predict these aggressive indicators of HCC before surgery to help patients develop more appropriate treatment options. Gadoxetic acid-enhanced magnetic resonance imaging (MRI) can accurately detect lesions and assess HCC characteristics. Previous studies have shown that some MRI features can predict the high pathologic grade, MVI positivity and CK19 expression in HCC. Nevertheless, no studies have used the same patient cohort to simultaneously predict the three aggressive features. Therefore, we systematically integrated the gadoxetic acid-enhanced MRI features that may be related to HCC aggressiveness, arranged in different sequences and phases, to conduct the study.Methods
This retrospective study included 147 patients with surgically confirmed HCCs who underwent gadoxetic-enhanced MRI. The lesions were evaluated quantitatively in terms of the relative enhancement ratio (RER), and qualitatively based on imaging features and clinical parameters. Logistic regression analyses were performed to investigate the value of these parameters in predicting the pathologic grade, MVI, and CK19 in HCC. Predictive factors for postoperative recurrence were determined using a Cox proportional hazards model.Results
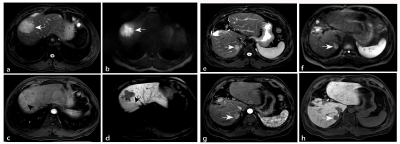
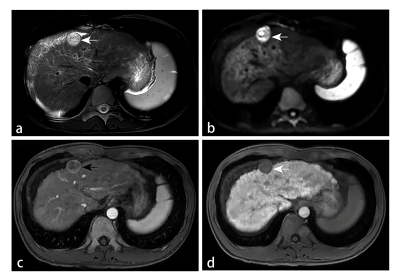
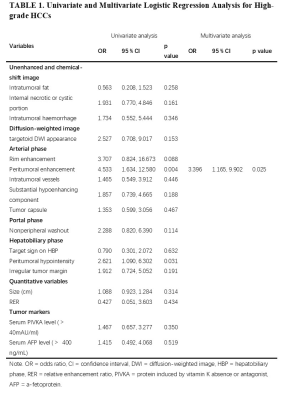
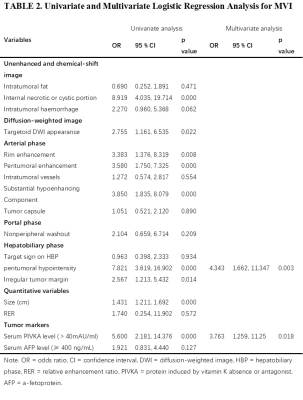
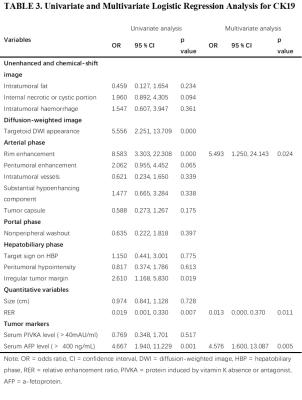
Peritumoral enhancement (odds ratio [OR], 3.396; p = 0.025) was an independent predictor of poor pathologic grades. Serum protein induced by vitamin K absence or antagonist (PIVKA) levels > 40 mAU/mL (OR, 3.763; p = 0.018) and peritumoral hypointensity (OR, 4.343; p = 0.003) were independent predictors of MVI. Predictors of CK19 included serum alpha-fetoprotein (AFP) levels > 400 ng/mL (OR, 4.576; p = 0.005), rim enhancement (OR, 5.493; p = 0.024), and lower RER (OR, 0.013; p = 0.011). Peritumoral hypointensity (hazard ratio [HR], 1.957; p = 0.027) and poor pathologic grades (HR, 2.339; p = 0.043) were independent predictors of recurrence.Discussion
Our study demonstrated that peritumoral enhancement in the AP was significant in predicting the poorer pathologic grade of HCC patients. The cause may be tumor invasion blocking tiny portal vein branches, reducing blood flow to this area and leading to preferential arterial supply or direct connection of the tumor sinusoids to the hepatic sinusoids. HBP peritumoral hypointensity and higher serum PIVKA levels (> 40 mAU/ml) were identified as significant variables for predicting MVI-positive HCCs. Peritumoral perfusion changes caused by MVI may downregulate the expression of OATP1B3 in hepatocytes around tumors, leading to an alteration of hepatic function and a reduction of HBP signal intensity. PIVKA, an angiogenic factor, is associated with aggressive behaviours in terms of proliferation, metastasis, and invasion. For the prediction of CK19, we found that rim enhancement in the AP, lower RER, and higher AFP levels (> 400 ng/ml) were significant independent risk factors. The imaging appearance of arterial rim enhancement is rare in common HCC but is frequently observed in cholangiocarcinoma or proliferative HCC. Several subtypes of proliferative HCC, including macrotrabecular HCC and scirrhous HCC, have been reported to have increased levels of CK19 expression. AFP was the earliest discovered and is the most widely used serum prognostic biomarker for HCC; it has been reported to be correlated with poor prognosis, high proliferation, and high angiogenesis in HCC. The low HBP SI of lesions relative to liver parenchyma was indicated to be associated with poor differentiation, and the underlying mechanism may be the decrease in expression of OATP1B3 with the progression of HCC. Therefore, we concluded that CK19 positive HCCs with low HBP enhancement might be more aggressive. The present study showed that postoperative recurrence was more frequently observed in HCCs with poorer pathologic grades or HBP peritumoral hypointensity. High pathologic grade is a known indicator of poor prognosis, this may be due to the correlation between poor differentiation and many metastasis- and invasion-associated genes in HCC. As mentioned above, HBP peritumoral hypointensity tends to predict MVI, whereas tumors with MVI are more prone to relapse. Consequently, HBP peritumoral hypointensity was highly suggestive of an increased risk of recurrence.Conclusion
We demonstrated the value of preoperative gadoxetic-enhanced MRI in predicting aggressive pathological features of HCC. Poor pathologic grades and peritumoral hypointensity may independently predict the recurrence of HCC.Acknowledgements
The study was approved by the ethics committee of the Affiliated Nantong Hospital 3 of Nantong University, and the requirement of informed consent was exempted.References
1. Villanueva, A. Hepatocellular Carcinoma. N Engl J Med 2019, 380 (15), 1450–1462.
2. Forner, A.; Reig, M.; Bruix, J. Hepatocellular Carcinoma. The Lancet 2018, 391 (10127), 1301–1314.
3. Palucka, A. K.; Coussens, L. M. The Basis of Oncoimmunology. Cell 2016, 164 (6), 1233–1247.
4. Jonas, S.; Bechstein, W. O.; Steinmüller, T.; Herrmann, M.; Radke, C.; Berg, T.; Settmacher, U.; Neuhaus, P. Vascular Invasion and Histopathologic Grading Determine Outcome after Liver Transplantation for Hepatocellular Carcinoma in Cirrhosis. Hepatology 2001, 33 (5), 1080–1086.
5. Pérez-Saborido, B.; de los Galanes, S. J.; Menéu-Díaz, J. C.; Romero, C. J.; Elola-Olaso, A. M.; Suárez, Y. F.; Valencia, V. B.; Moreno-González, E. Tumor Recurrence After Liver Transplantation for Hepatocellular Carcinoma: Recurrence Pathway and Prognostic Factors. Transplantation Proceedings 2007, 39 (7), 2304–2307.
6. Zhou, L.; Rui, J.-A.; Wang, S.-B.; Chen, S.-G.; Qu, Q. Clinicopathological Predictors of Poor Survival and Recurrence After Curative Resection in Hepatocellular Carcinoma Without Portal Vein Tumor Thrombosis. Pathol. Oncol. Res. 2015, 21 (1), 131–138.
7. Sumie, S.; Kuromatsu, R.; Okuda, K.; Ando, E.; Takata, A.; Fukushima, N.; Watanabe, Y.; Kojiro, M.; Sata, M. Microvascular Invasion in Patients with Hepatocellular Carcinoma and Its Predictable Clinicopathological Factors. Ann Surg Oncol 2008, 15 (5), 1375–1382.
8. Sumie, S.; Nakashima, O.; Okuda, K.; Kuromatsu, R.; Kawaguchi, A.; Nakano, M.; Satani, M.; Yamada, S.; Okamura, S.; Hori, M.; Kakuma, T.; Torimura, T.; Sata, M. The Significance of Classifying Microvascular Invasion in Patients with Hepatocellular Carcinoma. Ann Surg Oncol 2014, 21 (3), 1002–1009.
9. Zhang, X.; Li, J.; Shen, F.; Lau, W. Y. Significance of Presence of Microvascular Invasion in Specimens Obtained after Surgical Treatment of Hepatocellular Carcinoma. Journal of Gastroenterology and Hepatology 2018, 33 (2), 347–354.
10. Calderaro, J.; Ziol, M.; Paradis, V.; Zucman-Rossi, J. Molecular and Histological Correlations in Liver Cancer. Journal of Hepatology 2019, 71 (3), 616–630. 11. Llovet, J. M.; Villanueva, A.; Lachenmayer, A.; Finn, R. S. Advances in Targeted Therapies for Hepatocellular Carcinoma in the Genomic Era. Nat Rev Clin Oncol 2015, 12 (7), 408–424.
12. Chiang, D. Y.; Villanueva, A.; Hoshida, Y.; Peix, J.; Newell, P.; Minguez, B.; LeBlanc, A. C.; Donovan, D. J.; Thung, S. N.; Solé, M.; Tovar, V.; Alsinet, C.; Ramos, A. H.; Barretina, J.; Roayaie, S.; Schwartz, M.; Waxman, S.; Bruix, J.; Mazzaferro, V.; Ligon, A. H.; Najfeld, V.; Friedman, S. L.; Sellers, W. R.; Meyerson, M.; Llovet, J. M. Focal Gains of VEGFA and Molecular Classification of Hepatocellular Carcinoma. Cancer Research 2008, 68 (16), 6779–6788.
13. Zhuo, J.-Y.; Lu, D.; Tan, W.-Y.; Zheng, S.-S.; Shen, Y.-Q.; Xu, X. CK19-Positive Hepatocellular Carcinoma Is a Characteristic Subtype. J. Cancer 2020, 11 (17), 5069–5077.
14. Kim, H.; Choi, G. H.; Na, D. C.; Ahn, E. Y.; Kim, G. I.; Lee, J. E.; Cho, J. Y.; Yoo, J. E.; Choi, J. S.; Park, Y. N. Human Hepatocellular Carcinomas with “Stemness”-Related Marker Expression: Keratin 19 Expression and a Poor Prognosis. Hepatology 2011, 54 (5), 1707–1717.
15. Kim, T.-H.; Kim, S. Y.; Tang, A.; Lee, J. M. Comparison of International Guidelines for Noninvasive Diagnosis of Hepatocellular Carcinoma: 2018 Update. Clin Mol Hepatol 2019, 25 (3), 245–263.
16. Zhang, L.; Yu, X.; Wei, W.; Pan, X.; Lu, L.; Xia, J.; Zheng, W.; Jia, N.; Huo, L. Prediction of HCC Microvascular Invasion with Gadobenate-Enhanced MRI: Correlation with Pathology. Eur Radiol 2020, 30 (10), 5327–5336.
17. Lee, S.; Kim, S. H.; Lee, J. E.; Sinn, D. H.; Park, C. K. Preoperative Gadoxetic Acid–Enhanced MRI for Predicting Microvascular Invasion in Patients with Single Hepatocellular Carcinoma. Journal of Hepatology 2017, 67 (3), 526–534.
18. Huang, K.; Dong, Z.; Cai, H.; Huang, M.; Peng, Z.; Xu, L.; Jia, Y.; Song, C.; Li, Z.-P.; Feng, S.-T. Imaging Biomarkers for Well and Moderate Hepatocellular Carcinoma: Preoperative Magnetic Resonance Image and Histopathological Correlation. BMC Cancer 2019, 19 (1), 364.
19. Wei, H.; Jiang, H.; Liu, X.; Qin, Y.; Zheng, T.; Liu, S.; Zhang, X.; Song, B. Can LI-RADS Imaging Features at Gadoxetic Acid-Enhanced MRI Predict Aggressive Features on Pathology of Single Hepatocellular Carcinoma? European Journal of Radiology 2020, 132, 109312.
20. Hu, X.-X.; Wang, W.-T.; Yang, L.; Yang, Z.-X.; Liang, H.-Y.; Ding, Y.; Ji, Y.; Zeng, M.-S.; Rao, S.-X. MR Features Based on LI-RADS Identify Cytokeratin 19 Status of Hepatocellular Carcinomas. European Journal of Radiology 2019, 113, 7–14.
21. Choi, S.-Y.; Kim, S. H.; Park, C. K.; Min, J. H.; Lee, J. E.; Choi, Y.-H.; Lee, B. R. Imaging Features of Gadoxetic Acid–Enhanced and Diffusion-Weighted MR Imaging for Identifying Cytokeratin 19-Positive Hepatocellular Carcinoma: A Retrospective Observational Study. Radiology 2018, 286 (3), 897–908.
22. Wang, W.; Gu, D.; Wei, J.; Ding, Y.; Yang, L.; Zhu, K.; Luo, R.; Rao, S.-X.; Tian, J.; Zeng, M. A Radiomics-Based Biomarker for Cytokeratin 19 Status of Hepatocellular Carcinoma with Gadoxetic Acid–Enhanced MRI. Eur Radiol 2020, 30 (5), 3004–3014.
23. Fan, L.; Mac, M. T.; Frishberg, D. P.; Fan, X.; Dhall, D.; Balzer, B. L.; Geller, S. A.; Wang, H. L. Interobserver and Intraobserver Variability in Evaluating Vascular Invasion in Hepatocellular Carcinoma: Evaluation of Vascular Invasion in HCC. Journal of Gastroenterology and Hepatology 2010, 25 (9), 1556–1561.
24. Yang, F.; Wan, Y.; Xu, L.; Wu, Y.; Shen, X.; Wang, J.; Lu, D.; Shao, C.; Zheng, S.; Niu, T.; Xu, X. MRI-Radiomics Prediction for Cytokeratin 19-Positive Hepatocellular Carcinoma: A Multicenter Study. Front. Oncol. 2021, 11, 672126.
25. Kang, H.-J.; Kim, H.; Lee, D. H.; Hur, B. Y.; Hwang, Y. J.; Suh, K.-S.; Han, J. K. Gadoxetate-Enhanced MRI Features of Proliferative Hepatocellular Carcinoma Are Prognostic after Surgery. Radiology 2021, 300 (3), 572–582.
26. Weng, S.; Xu, X.; Li, Y.; Yan, C.; Chen, J.; Ye, R.; Zhu, Y.; Wen, L.; Hong, J. Quantitative Analysis of Multiphase Magnetic Resonance Images May Assist Prediction of Histopathological Grade of Small Hepatocellular Carcinoma. Ann Transl Med 2020, 8 (16), 1023–1023.
27. Min, J. H.; Lee, M. W.; Park, H. S.; Lee, D. H.; Park, H. J.; Lim, S.; Choi, S.-Y.; Lee, J.; Lee, J. E.; Ha, S. Y.; Cha, D. I.; Carriere, K. C.; Ahn, J. H. Interobserver Variability and Diagnostic Performance of Gadoxetic Acid–Enhanced MRI for Predicting Microvascular Invasion in Hepatocellular Carcinoma. Radiology 2020, 297 (3), 573–581.
28. Rhee, H.; Cho, E.-S.; Nahm, J. H.; Jang, M.; Chung, Y. E.; Baek, S.-E.; Lee, S.; Kim, M.-J.; Park, M.-S.; Han, D. H.; Choi, J.-Y.; Park, Y. N. Gadoxetic Acid-Enhanced MRI of Macrotrabecular-Massive Hepatocellular Carcinoma and Its Prognostic Implications. Journal of Hepatology 2021, 74 (1), 109–121.
29. Li, Y.; Chen, J.; Weng, S.; Sun, H.; Yan, C.; Xu, X.; Ye, R.; Hong, J. Small Hepatocellular Carcinoma: Using MRI to Predict Histological Grade and Ki-67 Expression. Clinical Radiology 2019, 74 (8), 653.e1-653.e9.
30. Dong, Z.; Huang, K.; Liao, B.; Cai, H.; Dong, Y.; Huang, M.; Zhou, X.; Jia, Y.; Xu, L.; Luo, Y.; Li, Z.-P.; Feng, S.-T. Prediction of Sorafenib Treatment–Related Gene Expression for Hepatocellular Carcinoma: Preoperative MRI and Histopathological Correlation. Eur Radiol 2019, 29 (5), 2272–2282.
31. Chernyak, V.; Fowler, K. J.; Kamaya, A.; Kielar, A. Z.; Elsayes, K. M.; Bashir, M. R.; Kono, Y.; Do, R. K.; Mitchell, D. G.; Singal, A. G.; Tang, A.; Sirlin, C. B. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289 (3), 816–830.
32. Aoki, T.; Nishida, N.; Ueshima, K.; Morita, M.; Chishina, H.; Takita, M.; Hagiwara, S.; Ida, H.; Minami, Y.; Yamada, A.; Sofue, K.; Tsurusaki, M.; Kudo, M. Higher Enhancement Intrahepatic Nodules on the Hepatobiliary Phase of Gd-EOB-DTPA-Enhanced MRI as a Poor Responsive Marker of Anti-PD-1/PD-L1 Monotherapy for Unresectable Hepatocellular Carcinoma. Liver Cancer 2021, 10 (6), 615–628.
33. Sasaki, R.; Nagata, K.; Fukushima, M.; Haraguchi, M.; Miuma, S.; Miyaaki, H.; Soyama, A.; Hidaka, M.; Eguchi, S.; Shigeno, M.; Yamashima, M.; Yamamichi, S.; Ichikawa, T.; Kugiyama, Y.; Yatsuhashi, H.; Nakao, K. Evaluating the Role of Hepatobiliary Phase of Gadoxetic Acid-Enhanced Magnetic Resonance Imaging in Predicting Treatment Impact of Lenvatinib and Atezolizumab plus Bevacizumab on Unresectable Hepatocellular Carcinoma. Cancers 2022, 14 (3), 827.
34. Tang, M.; Zhou, Q.; Huang, M.; Sun, K.; Wu, T.; Li, X.; Liao, B.; Chen, L.; Liao, J.; Peng, S.; Chen, S.; Feng, S.-T. Nomogram Development and Validation to Predict Hepatocellular Carcinoma Tumor Behavior by Preoperative Gadoxetic Acid-Enhanced MRI. Eur Radiol 2021, 31 (11), 8615–8627.
35. Fowler, K. J.; Burgoyne, A.; Fraum, T. J.; Hosseini, M.; Ichikawa, S.; Kim, S.; Kitao, A.; Lee, J. M.; Paradis, V.; Taouli, B.; Theise, N. D. Pathologic, molecular, and prognostic radiologic features of hepatocellular carcinoma. 41 (6), 21.
36. Aoki, T.; Nishida, N.; Kudo, M. Clinical Significance of the Duality of Wnt/β-Catenin Signaling in Human Hepatocellular Carcinoma. Cancers 2022, 14 (2), 444.
37. Ryu, T.; Takami, Y.; Wada, Y.; Tateishi, M.; Hara, T.; Yoshitomi, M.; Momosaki, S.; Yasumori, K.; Saitsu, H.; Okuda, K. A Clinical Scoring System for Predicting Microvascular Invasion in Patients with Hepatocellular Carcinoma Within the Milan Criteria. J Gastrointest Surg 2019, 23 (4), 779–787.
38. Yang, Y.; Li, G.; Lu, Z.; Liu, Y.; Kong, J.; Liu, J. Progression of Prothrombin Induced by Vitamin K Absence-II in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 726213.
39. Rhee, H.; An, C.; Kim, H.-Y.; Yoo, J. E.; Park, Y. N.; Kim, M.-J. Hepatocellular Carcinoma with Irregular Rim-Like Arterial Phase Hyperenhancement: More Aggressive Pathologic Features. Liver Cancer 2019, 8 (1), 24–40.
40. Galle, P. R.; Foerster, F.; Kudo, M.; Chan, S. L.; Llovet, J. M.; Qin, S.; Schelman, W. R.; Chintharlapalli, S.; Abada, P. B.; Sherman, M.; Zhu, A. X. Biology and Significance of Alpha-Fetoprotein in Hepatocellular Carcinoma. Liver International 2019, 39 (12), 2214–2229.
41. Morisaka, H.; Motosugi, U.; Ichikawa, S.; Ichikawa, T.; Kondo, T.; Onishi, H. Uptake of Gadoxetic Acid in Hepatobiliary Phase Magnetic Resonance Imaging and Transporter Expression in Hypovascular Hepatocellular Nodules. European Journal of Radiology 2021, 138, 109669.
42. Qin, X.; Yang, T.; Huang, Z.; Long, L.; Zhou, Z.; Li, W.; Gao, Y.; Wang, M.; Zhang, X. Hepatocellular Carcinoma Grading and Recurrence Prediction Using T1 Mapping on Gadolinium‑ethoxybenzyl Diethylenetriamine Pentaacetic Acid‑enhanced Magnetic Resonance Imaging. Oncol Lett 2019.
43. Zhou, L.; Rui, J.-A.; Zhou, W.-X.; Wang, S.-B.; Chen, S.-G.; Qu, Q. Edmondson-Steiner Grade: A Crucial Predictor of Recurrence and Survival in Hepatocellular Carcinoma without Microvascular Invasio. Pathology - Research and Practice 2017, 213 (7), 824–830.
44. Cha, D. I.; Jang, K. M.; Kim, S. H.; Kim, Y. K.; Kim, H.; Ahn, S. H. Preoperative Prediction for Early Recurrence Can Be as Accurate as Postoperative Assessment in Single Hepatocellular Carcinoma Patients. Korean J Radiol 2020, 21 (4), 402.
45. Feng, L.-H.; Dong, H.; Lau, W.-Y.; Yu, H.; Zhu, Y.-Y.; Zhao, Y.; Lin, Y.-X.; Chen, J.; Wu, M.-C.; Cong, W.-M. Novel Microvascular Invasion-Based Prognostic Nomograms to Predict Survival Outcomes in Patients after R0 Resection for Hepatocellular Carcinoma. J Cancer Res Clin Oncol 2017, 143 (2), 293–303.
46. Lee, S.; Kim, K. W.; Jeong, W. K.; Kim, M.-J.; Choi, G. H.; Choi, J. S.; Song, G.-W.; Lee, S.-G. Gadoxetic Acid–Enhanced MRI as a Predictor of Recurrence of HCC after Liver Transplantation. Eur Radiol 2020, 30 (2), 987–995.
Figures