2433
Magnetic Resonance Imaging of the Liver Microenvironment to Assess Hepatocellular Carcinoma Growth and Characteristics in Rat1Department of Convergence Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea, Republic of, 2Convergence Medicine Research Center, Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea, Republic of, 3Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea, Republic of, 4Medical Research Institute, Gangneung Asan Hospital, Gangneung-si, Gangwon-do, Korea, Republic of
Synopsis
Keywords: Liver, Tumor, Magnetic resonance imaging, Liver microenvironment, Nonalcoholic steatohepatitis, Hepatocellular carcinoma
In this study, a syngeneic orthotopic hepatocellular carcinoma (HCC) rat model with nonalcoholic steatohepatitis (NASH) was established to elucidate the role of the liver microenvironment using MRI. Our findings demonstrated that the HCC growth and progression were accelerated by the disease severity of NASH in the syngeneic orthotopic HCC rat model. This suggests that the liver microenvironment is important for HCC promotion.INTRODUCTION
Studies of the liver microenvironment are needed, as most cases of HCC develop in liver cirrhosis1-3. However, studies on monitoring the HCC growth and progression according to nonalcoholic fatty liver disease severity are limited. Therefore, we aimed to monitor through MRI the changes and characteristics of the tumor and whether promotes the progression of HCC following disease severity.MATERIALS & METHODS
To induce nonalcoholic steatohepatitis, MCD-diet was fed (MCD-groups) for 1 (mild group) and 4 weeks (severe group). Subsequently, SD-rats were injected with 30 μL of 0.5×10^6 N1S1-cells/Matrigel solution in the left liver lobe. MRI was performed using a 9.4-T magnet. The MRI parameters were as follows: (1) FFPD with TR/TE=3000/9.05 ms, FOV=50×50 mm2. The FFPD (%) was acquired using two proton density images, fat-saturated (water-only; A) and unsaturated (water+fat; B), calculated using: (B–A)/B×100 (%). (2) T1 map with TR/TE=4900/6.83 ms, averages=1, matrix size=96×96, FOV=50×50 mm2. (3) T2 map with TR/TE=3000/10 ms, averages=1, matrix size=96×96, FOV=50×50 mm2. (4) T2* map with TR/TE=800/2.56 ms, echo=6, flip angle=30°, averages=4, matrix size=128×128, FOV=50×50 mm2.RESULTS AND DISCUSSION
To investigate the effect of the liver microenvironment on HCC progression, we established an orthotopic HCC rat model with NASH induced using MCD-diet (Fig.1). Fat-saturated T1-weighted imaging confirmed that the increased disease severity remarkably accelerated the HCC progression in the severe group compared with the healthy liver group (Fig.2). Liver injury, inflammation, and steatosis were present as shown by increased serum AST and ALT levels and PDFF (%) in the MCD-groups. Compared with day 0, both serum AST and ALT levels increased in the MCD-groups 9 days after HCC implantation (Fig.3). Furthermore, to assess the microenvironment of HCC, H and E staining was performed and T1, T2, and T2* maps were obtained. In the MCD-groups, as the tumor size increased, necrotic area and hemorrhage were confirmed through H and E staining. The T1 values increased in the necrotic tumor center compared to the other regions. The T2 values were increased in the liver parenchyma following disease severity induced by the MCD-diet and decreased in the overall tumor. In the severe group, the tumor center showed decreased T2* value compared to the tumor margin. The increased T1 value and decreased T2* value of the tumor center might be due to hepatic necrosis and hemorrhage (Fig.4).CONCLUSION
Our data demonstrated that the liver microenvironment of NASH accelerated to HCC growth and progression following the disease severity. Moreover, the T1, T2, and T2* mapping have the potential to identify changes in the tumor center, margin, and liver regions.Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education [2022R1I1A1A01066589].References
1. Clavería-Cabello A, Matías M. A. HIF2α Activation in NASH: A New Force Pushing Toward HCC. Cell Mol Gastroenterol Hepatol. 2022;13(2):678-680.
2. Degroote, H, Lefere, S, et al. Characterization of the inflammatory microenvironment and hepatic macrophage subsets in experimental hepatocellular carcinoma models. Oncotarget. 2021;12(6):562-577.
3. European Association For The Study Of The Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236.
Figures
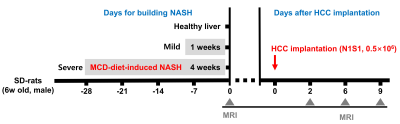
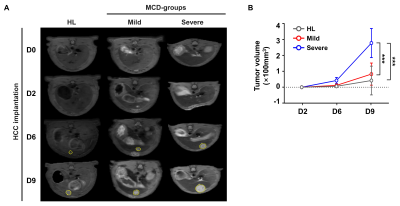
Fig.2. NASH induced by the MCD-diet accelerates HCC growth and progression.
(A) The representative fat-saturated T1-weighted images were obtained for a longitudinal study of the healthy liver and MCD-groups on day 0, 2, 6 and 9 after HCC implantation. (B) Tumor volume per liver (100 mm3) (*p<0.05, **p<0.01, ***p<0.001).
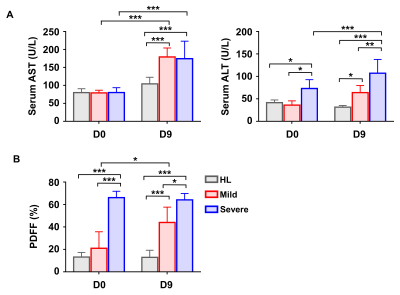
Fig.3. Comparison of the expression of serological markers and proton density fat fraction (PDFF) among the three groups on day 0 and 9.
(A) Serum levels of ALT and AST on day 0 and 9 after HCC implantation. (B) The FFPD (%) on day 0, 9 after HCC implantation (*p<0.05, **p<0.01, ***p<0.001).
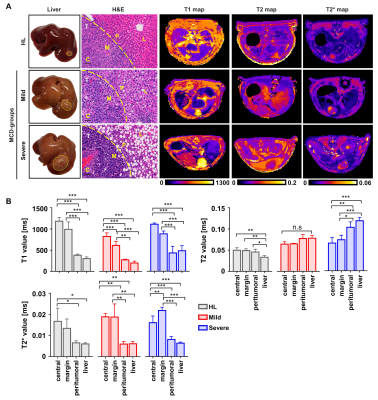
Fig.4. Changes in the several MRI parameters in the tumor and liver regions.
(A) The tumorigenesis was confirmed by H and E staining. The T1, T2, and T2* maps were obtained on day 9 after HCC implantation. T; tumor, C; tumor center, M; tumor margin, P; peritumoral region, L; liver parenchyma. (B) The T1, T2 and T2* values were measured among all regions on day 9 after HCC implantation (*p<0.05, **p<0.01, ***p<0.001).