2419
Exposure to Welding Fumes: Evaluating the metabolite-metal relationship1Purdue University, West Lafayette, IN, United States, 2School of Health Science, Purdue University, West Lafayette, IN, United States, 3Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, United States
Synopsis
Keywords: Data Analysis, Toxicity
Chronic exposure to Mn and Fe, through inhalation of welding fumes, is known to cause neurotoxicity. In this MRS study, we aim to determine if toenail Mn and Fe levels can be predictive of neurotoxicity identified through changes in GABA, GSH, and/or Glu concentrations. Significant GABA and GSH correlations with Fe indicate that toenail Fe concentrations can reflect decreased GABA and GSH levels in the welders’ brain. This emphasizes that Fe should not be ignored and might contribute to oxidative stress in Mn neurotoxicity.Introduction
High exposure to manganese (Mn) through inhalation of welding fumes has been shown to have a toxic effect to the human brain leading to a parkinsonism syndrome (manganism). Our group has demonstrated elevated thalamic γ-aminobutyric acid (GABA – the primary inhibitory neurotransmitter) levels, measured by edited magnetic resonance spectroscopy (MRS), in response to high exposure to Mn in welding fumes,1 which was found to be reversible if exposure is decreased.2 Furthermore, we have shown that toenail metal levels are a good biomarker for exposure to Mn and iron (Fe) over the past year.3,4 The current study investigates whether toenail Mn and Fe levels are predictive of brain GABA, Glutamate (Glu – the primary excitatory neurotransmitter), and glutathione (GSH – primary antioxidant in the brain) levels in two brain regions of Mn-exposed welders: the thalamus, a region responsible for relaying sensory and motor signals, and the cerebellum, the region that maintains balance, posture, and coordination. Due to the time it takes for toenails to grow, metal levels in toenail clippings have been shown to reflect an exposure window of 7-12 months prior to clipping,3 while brain Mn levels reflect exposure in more recent time windows (~0-3 months).1 Although changes in thalamic GABA with change in exposure has been demonstrated2, it is yet unknown what exposure window current metabolite levels would reflect.Methods
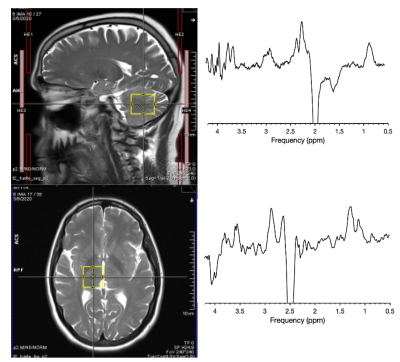
Stainless-steel welders (N=20 males, age range = 45±15 yrs) have been recruited from a local truck trailer manufacturer. Toenails have been collected every 3 months, with the initial clippings and MRS acquired at the first visit (baseline). GABA, Glu and GSH levels were acquired using HERMES (TE/TR:80/2000ms, 256 averages, 30x30x25mm3 VOI) in the thalamus and cerebellum of welders. Spectra were processed and analyzed using Gannet (v3.3).5 Toenail metal levels were cleaned and digested according to Ward et al.6 and analyzed via inductively coupled plasma-optical emission spectrometry (ICP-OES). Pearson correlations, with α=0.05 significance level, were run between toenail Mn and Fe and brain GABA, GSH, and Glu. All statistical analyses were performed in r-studio.Results
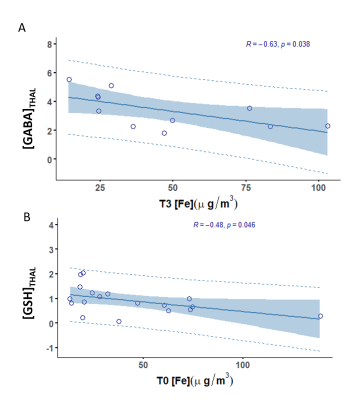
A preliminary analysis showed significant correlations between thalamic GABA and toenail Fe at 3 months (R=-0.63, p=0.038), and between thalamic GSH and toenail Fe at baseline (R=-0.48, p=0.046). However, no significant correlations between toenail Mn and GABA or Glu levels were obtained.Discussion and Conclusion
We have demonstrated GABA-Fe and GSH-Fe correlations, which indicates that increased toenail Fe concentrations may represent a decrease in thalamic GABA and GSH values. A significant GABA-Fe correlation at the second timepoint indicates that toenail Fe can be predictive of concurrent changes in thalamic GABA, caused by exposure 3-9 months before the MRI scan. Similarly, the significant GSH-Fe correlation at baseline may reflect increased oxidative stress due to elevated exposure 6-12 months prior to the MRI scan. Furthermore, this suggests that other metals, including Fe, that play a role in Mn homeostasis, should not be neglected in the study of Mn neurotoxicity.Acknowledgements
This study was supported by the R01 ES032478 and the International Manganese Institute.References
1. Ma RE, Ward EJ, Yeh CL, et al. Thalamic GABA levels and Occupational Manganese Neurotoxicity: Association with Exposure Levels and Brain MRI. Neurotoxicology. 2018;64:30. doi:10.1016/J.NEURO.2017.08.013
2. Edmondson DA, Ma RE, Yeh CL, et al. Reversibility of Neuroimaging Markers Influenced by Lifetime Occupational Manganese Exposure. doi:10.1093/toxsci/kfz174
3. Yeh CL, Johnson CB, Ma RE, Dharmadhikari S, Snyder S, Dydak U. Whole-Brain Visualization of Manganese Deposition in Welders. Proc Intl Mag Reson Med. 2017;25:3047.
4. Ward EJ, Edmondson DA, Nour MM, Snyder S, Rosenthal FS, Dydak U. Toenail Manganese: A Sensitive and Specific Biomarker of Exposure to Manganese in Career Welders. Ann Work Expo Heal. 2018;62(1):101. doi:10.1093/ANNWEH/WXX091
5. Chan, K. L., Oeltzschner, G., Saleh, M. G., Edden, R., & Barker, P. B. (2019). Simultaneous editing of GABA and GSH with Hadamard-encoded MR spectroscopic imaging. Magnetic resonance in medicine, 82(1), 21–32. https://doi.org/10.1002/mrm.27702
6. Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40(6):1445-1452. doi:10.1002/JMRI.24478
Figures