2414
Associated high-order resting-state functional connectivity network for diagnosis of schizophrenia1Department of Radiology, Xiang’an Hospital of Xiamen Uneversity,School of Medicine, Xiamen University, Xiamen, China
Synopsis
Keywords: Data Analysis, Brain Connectivity, high-order functional connectivity
Schizophrenia (SZ) is one of the most prevalent mental disorders; however, its accurate diagnosis is difficult in clinical practice. Currently, the underlying mechanism of SZ remains poorly understood. The associated higher-order functional connectivity (HOFC) which constructed based on the conventional FC is promising for understanding pathological changes of brain connectome. In our study,we found the model constructed with associated HOFC outperformed the model constructed with conventional FC. SZ-related brain regions were widely distributed in frontal, parietal, insula, occipital, subcortical, and limbic lobes, which are the core brain areas of the subcortical, fronto-parietal, sensorimotor, limbic, and default mode networks.Introduction
Schizophrenia (SZ) is one of the most prevalent mental disorders1; however, its accurate diagnosis is difficult2,3. Currently, the underlying mechanism of SZ remains poorly understood3-5. Resting-state functional MRI is one of the most commonly used non-invasive techniques in neuroimaging6-8. The functional connectivity (FC)7,9, as one of the most commonly used rs-fMRI measurements, has been widely used in neuropsychiatric disorders. The associated higher-order functional connectivity (HOFC) which constructed based on the conventional FC is promising for understanding pathological changes of brain connectome 10,11. This study aimed to explore the value of the associated HOFC in distinguishing SZ and healthy controls (HCs) and the potential brain functional network abnormalities in SZ.Methods
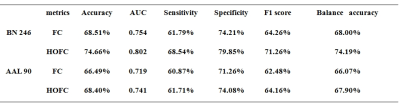
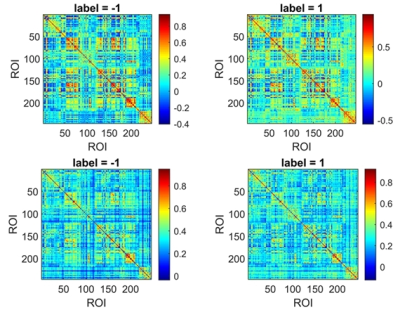
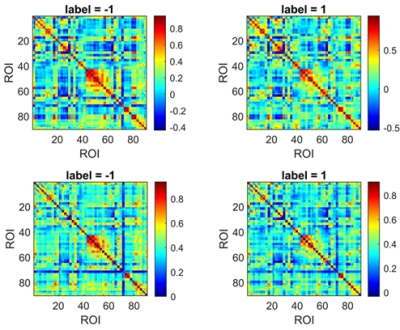
Fifty-six patients with SZ and 66 age- and sex-matched HCs were included in our study. All subjects' MRI data were routinely preprocessed; the conventional FC and associated HOFC were constructed using the Brainnetome 246 atlas. The t-tests (P = 0.05) and least absolute shrinkage and selection operator (λ= 0.20) were used for feature selection, and 10-fold cross-validation (repeated 100 times) 7,12 with Gaussian radial basis function support vector machine was applied for model training and evaluation. We compared the performance of the models based conventional FC and associated HOFC. The features with absolute value of weights greater than 30% of the maximum absolute weight in the 1000 iterations were selected as "discriminative features"13-15. The above analysis procedure was repeated using automated anatomical labeling (AAL) 90 atlas to further validate our results.Results
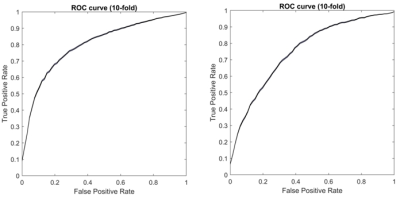
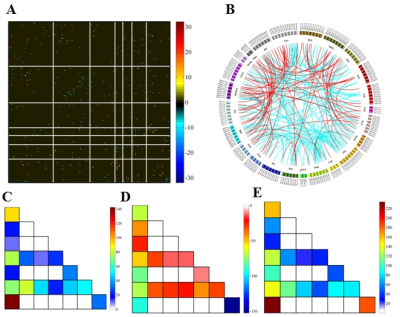
When using the Brainnetome 246 atlas, the average AUCs and accuracies of the model based on associated HOFC and conventional FC were 0.802, 74.66%, and 0.754, 68.51%, respectively. The model constructed with associated HOFC outperformed the model constructed with conventional FC. Similar results were obtained using the AAL 90 atlas (associated HOFC: AUC, 0.741, accuracy, 68.40%; conventional FC, AUC, 0.719, accuracy, 66.49%). The brain regions we identified as "discriminative features" were widely distributed in frontal, parietal, insula, occipital, subcortical, and limbic lobes, which are the core brain areas of the subcortical, fronto-parietal, sensorimotor, limbic, and default mode networks.Conclusion
Compared with conventional FC, associated HOFC can achieve better performance in diagnosing SZ, indicating that associated HOFC may better characterize abnormal brain function network in SZ, and provide help for a more comprehensive understanding of the pathological mechanism of SZ.Keywords
Schizophrenia, Functional connectivity, Machine learning, Support vector machineAcknowledgements
This study was supported by the Scientiêc Research Foundationfor Advanced Talents, Xiang’an Hospital of Xiamen University(No.PM201809170011).References
1. Charlson FJ, Ferrari AJ, Santomauro DF, et al. Global Epidemiology and Burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016. Schizophr Bull. Oct 17 2018;44(6):1195-1203. doi:10.1093/schbul/sby058
2. Shi D, Li Y, Zhang H, et al. Machine Learning of Schizophrenia Detection with Structural and Functional Neuroimaging. Dis Markers. 2021;2021:9963824. doi:10.1155/2021/9963824
3. Liu Z, Palaniyappan L, Wu X, et al. Resolving heterogeneity in schizophrenia through a novel systems approach to brain structure: individualized structural covariance network analysis. Mol Psychiatry. Dec 2021;26(12):7719-7731. doi:10.1038/s41380-021-01229-4
4. Wulff S, Nielsen MO, Rostrup E, et al. The relation between dopamine D2 receptor blockade and the brain reward system: a longitudinal study of first-episode schizophrenia patients. Psychol Med. Jan 2020;50(2):220-228. doi:10.1017/S0033291718004099
5. Jiang Y, Yao D, Zhou J, et al. Characteristics of disrupted topological organization in white matter functional connectome in schizophrenia. Psychol Med. Sep 3 2020:1-11. doi:10.1017/S0033291720003141
6. Qiu S, Joshi PS, Miller MI, et al. Development and validation of an interpretable deep learning framework for Alzheimer's disease classification. Brain. Jun 1 2020;143(6):1920-1933. doi:10.1093/brain/awaa137
7. Lin H, Cai X, Zhang D, Liu J, Na P, Li W. Functional connectivity markers of depression in advanced Parkinson's disease. Neuroimage Clin. 2020;25:102130. doi:10.1016/j.nicl.2019.102130
8. Zhao K, Ding Y, Han Y, et al. Independent and reproducible hippocampal radiomic biomarkers for multisite Alzheimer’s disease: diagnosis, longitudinal progress and biological basis. Science Bulletin. 2020;65(13):1103-1113. doi:10.1016/j.scib.2020.04.003 9. Shao J, Dai Z, Zhu R, et al. Early identification of bipolar from unipolar depression before manic episode: Evidence from dynamic rfMRI. Bipolar Disord. Dec 2019;21(8):774-784. doi:10.1111/bdi.12819 10. Zhang H, Chen X, Zhang Y, Shen D. Test-Retest Reliability of "High-Order" Functional Connectivity in Young Healthy Adults. Front Neurosci. 2017;11:439. doi:10.3389/fnins.2017.00439
11. Zhang Y, Zhang H, Chen X, Lee SW, Shen D. Hybrid High-order Functional Connectivity Networks Using Resting-state Functional MRI for Mild Cognitive Impairment Diagnosis. Sci Rep. Jul 26 2017;7(1):6530. doi:10.1038/s41598-017-06509-0
12. Wottschel V, Chard DT, Enzinger C, et al. SVM recursive feature elimination analyses of structural brain MRI predicts near-term relapses in patients with clinically isolated syndromes suggestive of multiple sclerosis. Neuroimage Clin. 2019;24:102011. doi:10.1016/j.nicl.2019.102011
13. Yin T, Sun R, He Z, et al. Subcortical-Cortical Functional Connectivity as a Potential Biomarker for Identifying Patients with Functional Dyspepsia. Cereb Cortex. Dec 10 2021;doi:10.1093/cercor/bhab419
14. Ecker C, Marquand A, Mourao-Miranda J, et al. Describing the brain in autism in five dimensions--magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. J Neurosci. Aug 11 2010;30(32):10612-23. doi:10.1523/JNEUROSCI.5413-09.2010
15. Yang X, Hu X, Tang W, et al. Multivariate classification of drug-naive obsessive-compulsive disorder patients and healthy controls by applying an SVM to resting-state functional MRI data. BMC Psychiatry. Jul 5 2019;19(1):210. doi:10.1186/s12888-019-2184-6
Figures