2413
MRI biomarkers of neurofluid production and egress provide support for aberrations in the neurofluid circuit in Huntington’s disease1Department of Neurology, Vanderbilt University Medical Center, Nashville, TN, United States, 2Department of Radiology, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Keywords: Data Analysis, Neurodegeneration
The overall goal of this work is to apply novel MRI methods, sensitive to CSF production and clearance, to test fundamental hypotheses regarding altered neurofluid circulation in patients with Huntington’s disease (HD). In participants with HD and healthy age- and sex-matched controls, we applied multi-modal MRI to investigate deviations of neuroimaging biomarkers of the neurofluid circuit at different locations: choroid plexus, cerebral aqueduct, and PSD. Analyses show a significant increase in ChP volume and PSD volume, and a decrease in ChP perfusion and CSF net flow, in HD participants relative to healthy controls.Introduction
The overall goal of this work is to apply novel MRI methods, sensitive to CSF production and clearance, to test fundamental hypotheses regarding altered neurofluid circulation in patients with Huntington’s disease (HD). HD is an autosomal dominant, neuro-degenerative disorder characterized by an expansion of the CAG repeat in the huntingtin gene, accompanied by motor, cognitive, and psychiatric symptoms. CSF circulation has recently been suggested to have fundamental relevance to understanding both mechanisms of mutant huntingtin (mHTT) protein retention as well as to emerging, intrathecally administered treatments1. Recent studies have indicated that mHTT is cleared from CSF through active mechanisms2,3, and altered neurofluid circulation may have relevance to mHTT retention and symptomatology4. Relevant to emerging models of neurofluid circulation is (i) CSF production in the choroid plexus complexes and (ii) CSF flow through the cerebral aqueduct, and, now following suggestions of glymphatic neurofluid circulation, (iii) CSF egress along parasagittal dura (PSD) spaces which have been hypothesized to harbor lymphatic collectors5–7. While many of these pathways have been investigated in other conditions, including aging and proteinopathies affecting older adults8,9, these pathways have not been investigated in patients with HD. In this work, we re-parameterize arterial spin labeling (ASL) and phase contrast sequences to evaluate ChP perfusion and aqueductal CSF flow, respectively. We complement these methods with novel deep learning algorithms applied to high spatial resolution structural imaging of PSD spaces to evaluate alterations of neurofluid production and egress in HD.Methods
60 participants (25 HD participants and 38 age- and sex-matched controls), provided informed, written consent and were scanned at 3T (Philips) using dual channel body coil transmission and 32-channel phased array reception. Clinical.Number of CAG repeats, CAG-age-product (CAP) score (a measure of increasing disease exposure), and manifest status were quantified from CSF assays and neurological exam. Acquisition. 3D T1-weighted (T1w; spatial-resolution=1x1x1mm), 3D T2-weighted (T2w; spatial-resolution=0.78x0.78x0.78mm), 2D T2-FLAIR (spatial-resolution = 0.57x0.57x5mm), 2D pCASL (spatial-resolution=3x3x5mm), and 2D ECG-calibrated phase contrast for CSF flow (venc=12cm/s; spatial-resolution=0.58x0.58x4mm). Analysis. Total intracranial volume (TICV) was quantified using AssemblyNet10 from T1w acquisitions. T1w and T2-FLAIR scans were supplied to a machine learning algorithm to segment ChP in the atria of the lateral ventricles11. Resulting ChP masks were used to estimate ChP volume and perfusion from pCASL using algorithms presented in the recent literature9. CSF net flow was computed as the difference of cranial and caudal flow as previously reported9. PSD volume, an anatomical location of CSF egress, was quantified using a deep-learning algorithm applied on T2w scans12. All segmentation maps were validated by a board-certified neuroradiologist (experience=13 years). Hypothesis testing. A multi-linear regression model was applied to test the hypothesis that HD patients have unique function and anatomical markers of neurofluid flow calculated from the above methods, compared to healthy controls. ChP volume and perfusion, CSF net flow in the cerebral aqueduct, and PSD volume were used in separate regression analyses as independent variables, whereas age, sex, and group were included as dependent variables. Statistical significance criteria: p<0.05.Results
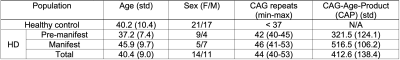
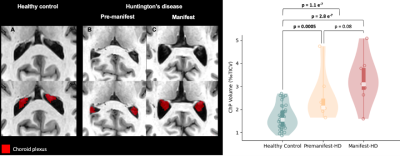
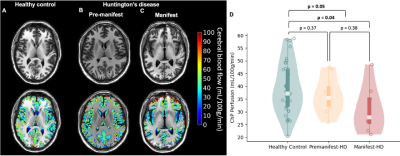
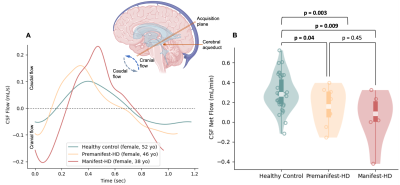
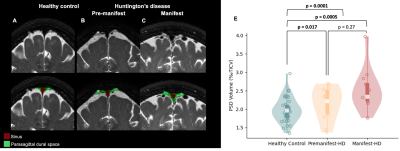
Table 1 summarizes the demographic and clinical criteria for the HD cohort, which comprised 12 manifest and 13 pre-manifest participants. ChP volume. ChP volume was observed to be elevated in HD cohorts relative to healthy participants (p<0.001), as well as between healthy and premanifest-HD (p<0.001) and healthy and manifest-HD (p<0.001) (Fig. 1). ChP perfusion. ChP perfusion was reduced between healthy and manifest-HD only (p=0.02) (Fig. 2).CSF Net Flow. CSF net flow was reduced for HD participants relative to healthy participants (p<0.001), manifest-HD relative to healthy participants (p-value=0.004), and premanifest-HD relative to healthy participants (p-value=0.001) (Fig. 3). PSD volume. Using volumetric assessment of the PSD after normalization for TICV (Fig. 4), PSD volume was significantly hypertrophied for the HD cohort relative to healthy participants (p<0.001), manifest-HD relative to healthy participants (p-value=0.004), and premanifest HD relative to healthy participants.Discussions and Conclusions
In participants with HD and healthy age- and sex-matched controls, we applied multi-modal MRI to investigate deviations of neuroimaging biomarkers of the neurofluid circuit at different locations: choroid plexus, cerebral aqueduct, and PSD. Analyses show a significant increase in ChP volume and PSD volume, and a decrease in ChP perfusion and CSF net flow, in HD relative to healthy participants. These results can be interpreted alongside the growing literature describing CSF circulation in HD. For instance, recent work in rodents has provided support for intracellular mHTT being cleared to the CSF along active mechanisms3, and a recent study in humans has provided evidence of perivascular space enlargement in HD, which may have relevance to aberrant neurofluid flow along perivascular spaces4. Our findings also extend recent work quantifying CSF dynamics in the cerebral aqueduct13, which found a trend of reduced CSF dynamic flow in HD; by tripling the sample size in our study, we find net CSF flow to be reduced in HD. Interpreting this finding with the reduction of ChP perfusion could indicate an abnormal reduction of CSF production in HD. Finally, it is noteworthy that our findings are consistent with trends seen in CSF production and egress in aging8,9, and the patterns of changes observed in HD are consistent with a model of accelerated aging.Acknowledgements
This study has been supported in part by the National Institute of Health (NIH) through grant numbers K24-AG064114 and R01AG062574, the Department of Defense (DoD) W81XWH-19-1-0812, and the Huntington's Disease Society of America (HDSA) HD Human Biology Project Fellowship.References
1. Stoker, T. B., Andresen, K. E. R. & Barker, R. A. Hydrocephalus Complicating Intrathecal Antisense Oligonucleotide Therapy for Huntington’s Disease. Mov. Disord. 36, 263–264 (2021).
2. Caron, N. S. et al. Potent and sustained huntingtin lowering via AAV5 encoding miRNA preserves striatal volume and cognitive function in a humanized mouse model of Huntington disease. Nucleic Acids Res. 48, 36–54 (2020).
3. Caron, N. S. et al. Mutant Huntingtin Is Cleared from the Brain via Active Mechanisms in Huntington Disease. J. Neurosci. 41, 780–796 (2021).
4. Chan, S. T., Mercaldo, N. D., Ravina, B., Hersch, S. M. & Rosas, H. D. Association of Dilated Perivascular Spaces and Disease Severity in Patients With Huntington Disease. Neurology 96, e890–e894 (2021).
5. Jessen, N. A., Munk, A. S. F., Lundgaard, I. & Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015 4012 40, 2583–2599 (2015).
6. Ringstad, G. & Eide, P. K. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat. Commun. 11, 1–9 (2020).
7. Park, M., Park, J. P., Kim, S. H. & Cha, Y. J. Evaluation of dural channels in the human parasagittal dural space and dura mater. Ann. Anat. - Anat. Anzeiger 244, 151974 (2022).
8. Hett, K. et al. Parasagittal dural space and cerebrospinal fluid (CSF) flow across the lifespan in healthy adults. Fluids Barriers CNS 1–33 (2022) doi:10.1186/s12987-022-00320-4.
9. Eisma, J. et al. Choroid plexus perfusion and bulk cerbrospinal fluid flow across the adult lifespan. J. Cereb. Blood Flow Metab. (2022) doi:10.1177/0271678X221129101.
10. Coupé, P. et al. AssemblyNet: A large ensemble of CNNs for 3D Whole Brain MRI Segmentation. Neuroimage219, 117026 (2019).
11. Zhao, L., Feng, X., Meyer, C. H. & Alsop, D. C. Choroid Plexus Segmentation Using Optimized 3D U-Net. in Proceedings - International Symposium on Biomedical Imaging 381–384 (IEEE Computer Society, 2020).
12. Ronneberger, O., Fischer, P. & Brox, T. U-net: Convolutional networks for biomedical image segmentation. Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics) 9351, 234–241 (2015).
13. Rodrigues, F. B. et al. Cerebrospinal fluid flow dynamics in Huntington’s disease evaluated by phase contrast MRI. Eur. J. Neurosci. 49, 1632–1639 (2019).
Figures