2387
Flow-suppressed 2D Spin-Echo EPI with high B1-insensitivity using Hyperbolic Secant Pulses1Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of, 2Department of Radiology, Seoul National University Hospital and College of Medicine, Seoul, Korea, Republic of, 3Department of Radiology, Center for Magnetic Resonance Research, Minneapolis, MN, United States, 4Department of Intelligent Precision Healthcare Convergence, Sungkyunkwan University, Suwon, Korea, Republic of
Synopsis
Keywords: Pulse Sequence Design, Diffusion/other diffusion imaging techniques, Hyperbolic-Secant Pulse
Blood flow artifacts sometimes occur in 2D spin-echo sequence despite its intrinsic flow-suppression effect. Pre-saturation pulses have widely been used for flow-suppression, but it has several disadvantages such as requirement of additional RF pulses and being not effective at suppressing relatively slow blood flow signal. In this study, we propose a more effective flow-suppressed spin-echo sequence using hyperbolic-secant (HS) pulses for π/2-excitation and π-refocusing. The proposed method was applied to 2D spin-echo diffusion EPI for liver imaging.Introduction
Diffusion-weighted imaging (DWI) is widely used in clinical applications due to its high sensitivity to Brownian motion of water molecules. Liver DWI using a spin-echo diffusion EPI sequence is one of the popular clinical applications of DWI. However, liver DWI suffers from cardiac and respiratory motions, particularly in left lobe of the liver1. To reduce motion-induced artifacts, motion-compensated diffusion-weighted gradients with gradient moment nulling (e.g. bipolar diffusion-weighted gradients) have been used, and some optimization techniques have also been recently proposed to reduce the minimum TE2-4. However, motion-compensated gradients can make moving blood spins to be in-phase, so bright blood signals are often detected in DWI. Several methods have been proposed to eliminate these bright blood signals. Van et al. applied monopolar diffusion-weighted gradient along the anterior-posterior direction with bipolar diffusion-weighted gradients applied in other directions5, and Zhang et al. proposed a motion-robust and blood-suppressed DWI sequence with a moderate first-moment motion sensitive (M1) value4. However, since these techniques use moderate M1 values, they provide only partial blood suppression and motion compensation. In this study, we propose a flow-suppressed 2D spin-echo EPI sequence that uses hyperbolic-secant (HS) pulses for both π/2-excitation and π-refocusing, with high B1-insensitivity due to the adiabatic property of the π refocusing HS pulse. The performance of this sequence was demonstrated by theory, simulation, and liver imaging.Methods
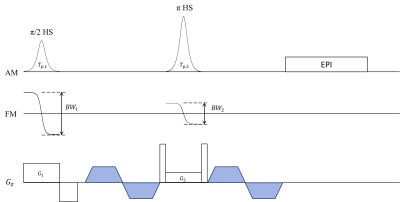
Ro et al. proposed a flow-suppression technique using tailored π/2 and π RF pulses that could produce opposing quadratic phases in a spin-echo sequence6. On the other hand, Park et al. demonstrated that when HS pulses are used for both π/2-excitation and π-refocusing in a spin-echo sequence, opposing quadratic-function-like non-linear phase distributions would be generated and could be canceled in specific conditions7. We used one of these conditions to achieve flow suppression, i.e., Tp,1 = Tp,2 , BW1 = 2BW2 , G1 = 2G2, where Tp is a pulse duration, BW is a pulse bandwidth, G is a slice-selection gradient. Subscripts 1 and 2 denote π/2-excitation and π-refocusing, respectively (Fig. 1). First, numerical simulations were performed to investigate flow suppression as a function of flow velocity and pulse parameters, using HS pulses with Tp,1 = Tp,2 = 5.12 ms, BW1/2π = 2BW2/2π = 4.00 kHz. Then, human liver images were acquired with the proposed method using a body coil on a 3T scanner (Prisma, Siemens, Erlangen, Germany). Scan parameters were as follows: TR/TE = 1200/80 ms, FOV = 280×280 mm2, matrix size = 128×128, slice thickness = 6 mm, b(average) = 50(2) s/ mm2, readout-BW = 1502 Hz/px, acceleration factor = 2, and fat suppression = Spectral Attenuated Inversion-Recovery (SPAIR)8. HS pulses with Tp,1 = Tp,2 = 5.12 ms, BW1/2π = 2BW2/2π = 4.00 kHz. For comparison, same experiments were performed using sinc pulses. In all human experiments, prospective respiratory gating was used.Results
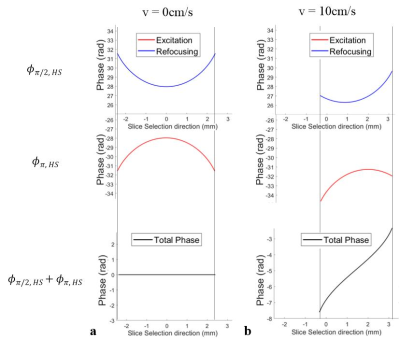
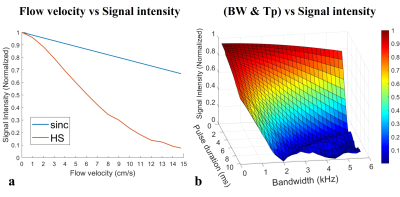
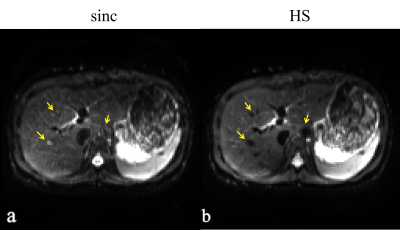
Figure 2a shows the simulation results that non-linear phase profiles are fully compensated for static spins. In contrast, for moving spins, a nearly linear phase distribution is formed due to the shifted quadratic phase distribution with opposite polarity (Fig. 2b), resulting in signal loss within a slice (Fig. 3a). Signal intensity for a given velocity decreased as pulse duration and bandwidth increased (Fig. 3b, flow velocity = 10 cm/s). In Fig. 4, thanks to the adiabatic π refocusing HS pulse, human liver images acquired with HS pulses showed better image quality than sinc pulses in terms of SNR, especially in the abdominal periphery. Moreover, moving blood signals were suppressed well, especially in the abdominal aorta and right branch of the portal vein (yellow arrows).Discussions
The proposed flow-suppression spin-echo diffusion EPI sequence using HS pulses was not only effective to suppress blood flow in the liver, especially prominent in the abdominal aorta, but also able to provide better image quality due to less sensitivity to B1 inhomogeneity. An additional advantage is that the parameters can be optimized to target a specific velocity of blood flow. When it is targeted to suppress slow blood flow (e.g., 2.5cm/s), it can also be more robust to B0 inhomogeneity effects due to relatively high RF pulse bandwidth (e.g., BW1/2π = 2BW2/2π = 6.13 kHz). From a practical viewpoint, the proposed method does not require any additional RF pulses or gradients, except for replacing the RF pulses with HS pulses. A limitation of the proposed method at this stage is that flow suppression is achieved only in the slice-selection direction.Conclusions
A flow-suppressed 2D spin-echo EPI sequence with high B1-insensitivity was proposed using hyperbolic secant pulses and successfully demonstrated by simulations and human liver imaging. Although we presented the method in spin-echo diffusion EPI, the technique can also be applied to any types of spin-echo sequences.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT): NRF-2020R1A2B5B02002676, NRF-2021R1A4A5032806.References
1. Murphy P, Wolfson T, Gamst A, Sirlin C, Bydder M. Error model for reduction of cardiac and respiratory motion effects in quantitative liver DW-MRI. J Magn Reson Imaging. 2013;70:1460–1469.
2. Aliotta E, Wu HH, Ennis DB. Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion-compensated diffusion-weighted MRI. Magn Reson Med. 2017;77:717–729.
3. Peña Nogales O, Zhang Y, Wang X, et al. Optimized Diffusion‐Weighting Gradient Waveform Design (ODGD) formulation for motion compensation and concomitant gradient nulling. Magn Reson Med. 2019;81:989–1003.
4. Zhang Y, Peña‐Nogales Ó, Holmes JH, Hernando D. Motion‐robust and blood‐suppressed M1‐optimized diffusion MR imaging of the liver. Magn Reson Med. 2019;00:1–10.
5. Van AT, Cervantes B, Ogino T, et al. Partial velocity‐compensated diffusion encoding for combined motion compensation and residual vessel signal suppression in liver DWI. In Proceedings of the 26th Annual Meeting of ISMRM, Paris, France; 2018.
6. Ro, Y. M, Cho, Z. H. A novel flow-suppression technique using tailored RF pulses. Magn Reson Med. 1993;29(5):660–666.
7. Park J-Y et al, Spin-echo MRI using π/2 and π hyperbolic secant pulses. Magn Reson Med. 2006;55:848-857.
8. Lauenstein TC, Sharma P, Hughes T, Heberlein K, Tudorascu D, Martin DR. Evaluation of optimized inversion-recovery fat-suppression techniques for T2-weighted abdominal MR imaging. J Magn Reson Imaging 2008;27(6):1448–1454.
Figures