2386
Off-resonance and B1+ Resilience of Relaxation Along a Fictitious Field (RAFF) pulses
1Department of Imaging Physics, Delft University of Technology, Delft, Netherlands, 2Delft University of Technology, Delft, Netherlands, 3HollandPTC, Delft, Netherlands
Synopsis
Keywords: Pulse Sequence Design, Cartilage, RAFF
Spin-lock relaxation times can provide valuable markers for pathological remodelling, but their acquisition with constant amplitude spin-lock pulses is limited by SAR and sensitive to field inhomogeneities. Relaxation along a Fictitious Field (RAFF) can be used to measure spin-lock relaxation with reduced SAR burden. In this work, we evaluate the resilience of RAFF against field B0 and B+1 field inhomogeneities. Yet another RAFF (yaRAFF) pulse is introduced for fictitious field spin-locking with increased effective field strength. yaRAFF shows >5.9% reduced susceptibility for field inhomogeneities compared with RAFF in simulations and phantom measurements. In vivo images of yaRAFF in the calf show higher precision for small off-resonances, the mapping quality for large off-resonance is maintained for in phantom and in vivo TRAFF2 times at 3T.
Introduction
Spin-lock (SL) relaxation times (T1ρ) have been proposed as a promising biomarker for multiple diseases, including myocardial fibrosis1 and cartilage degeneration2. However, quantification of T1ρ based on constant amplitude SL pulses implies high SAR burden and is susceptible to field inhomogeneities. Relaxation Along a Fictitious Field (RAFF) was developed to overcome SAR limitations of conventional SL and showed comparable sensitivity to slow molecular motion in tissue3. Sensitivity to off-resonances is reduced with RAFF, but remains a limitation. This hinders quantification quality and hampers clinical applications. In this work, the susceptibility of RAFF pulses to field inhomogeneities is investigated and an improved pulse for fictitious field SL relaxometry with increased resilience against off-resonance and B+1 inhomogeneities is proposed.Methods
The performances of conventional spin-lock (RefSL)4, RAFF, and yet another RAFF (yaRAFF) pulses are investigated using Bloch simulations and phantom acquisitions for varying B0 and B+1 inhomogeneities. For yaRAFF, as opposed to RAFF, the fictitious field strength is decoupled from the effective field strength, leading to an RF-shape with a weighted sum of sine and cosine (Fig1C). Simulations were used to find optimal weighting parameter β and ωmax values for yaRAFF (Fig2). Different measures were used to quantify pulse performance in the optimization: 1) The averaged preparation efficiency Mz/M0 over the region of interest (ROI) (η1,Δω1)∈[0.5,1]x[-200,200] Hz, here η1 is the ratio between effective and nominal B+1 power and Δω1 the off-resonance. 2) The magnetization path length as a measure of spin-locking efficiency (Fig2B-C). In phantom validation experiments were conducted for η1=[0.2,0.4,…,1] with Δω1=[-200,-180,...,200]Hz for RefSL, and Δω1=[-400,-380,...,400]Hz for RAFF and yaRAFF (Fig3). The FWHM was defined as the frequency width at Mz/M0=0.5.For mapping quality assessment, in vivo TRAFF maps of the calf were acquired at nine off-resonances (Δω1=[-200,-150,…,200]Hz) in two healthy subjects (male and female, 26±1y, Fig4-5).
Mapping was performed at 3T (Philips Ingenia) acquiring 4 TRAFF-prepared bSSFP images (max RF power=13.5μT, pulse duration=2.84ms, preparation duration=0,28,57,85ms) and a saturation image (Fig1A). All scans shared the following imaging parameters: resolution=2x2mm2, slice-thickness=8mm, FOV=181x181mm2, flip-angle=700, TE/TR=0.83/2.05ms. Maps were obtained by fitting a three-parameter model. The coefficient of variation (CoV) within manually-drawn ROIs was used to assess precision (Fig5).
Results
Simulation shows increased averaged Mz for increasing peak frequency with the yaRAFF-pulse (Fig2A). At 500Hz (maximum allowed frequency), the averaged Mz showed an increasing trend for smaller β, while the magnetization path length decreased. The optimal β=0.62 resulted in higher performance (0.973) compared with RAFF (0.919) (Fig2B).In simulations, the FWHM of the off-resonance response was 320±8, 448±8, and 668±8Hz, for RefSL, RAFF, and yaRAFF, respectively. Phantom experiments show close agreement with the simulations (Fig3), yielding an off-resonance FWHM of 240±20, 500±25, and 700±50 Hz for RefSL, RAFF, and yaRAFF, respectively.
In-vivo magnitude baseline images acquired in one healthy subject are shown in Fig4 for RefSL, RAFF, and yaRAFF at nine off-resonances (Δω1=±200Hz). RefSL shows poor resilience against off-resonance artifacts (Fig4A). Across several off-resonances yaRAFF achieves more homogeneous signal intensities compared with RAFF (Fig4B-C). For quantitative mapping, RefSL shows poor precision in the presence of off-resonances with a CoV>15%, while RAFF and yaRAFF yield substantially improved off-resonance resilience, with an average CoV of 3.1% and 2.9%, respectively (Fig5).
Discussion
In this work, we investigate the susceptibility of fictitious field SL pulses against field inhomogeneities. Simulations and phantom experiments show good resilience against B+1 inhomogeneities but moderate susceptibility to off-resonance. An adapted pulse, yaRAFF, is proposed to improve off-resonance sensitivity. Phantom and in vivo results show 40% and 191% increased off-resonance tolerance with yaRAFF compared to RAFF and continuous-amplitude spin-lock, respectively.RAFF pulses operate in a sub-adiabatic regime, where the effective field strength and fictitious field strength are matched. yaRAFF is based on relaxing this condition to yield an amplitude and frequency-modulated pulse, which operates closer to the adiabatic conditions. Thus, increased off-resonance performance is achieved, while maintaining a spin-lock at an effective field with comparable strength.
The implemented yaRAFF had an alternation in the frequency modulation function, leading to a slight off-center frequency shift. Thus, Increased resilience against positive off-resonance compared with negative off-resonance is observed. Consequently, for in vivo application, the pulse is best played at a positive off-resonance shift, to center the off-resonance tolerance.
Image quality, as well as the precision of quantitative spin-lock mapping, obtained with the three pulses showed strong susceptibility to off-resonances. yaRAFF achieved the largest resilience, to off-resonances in vivo, and the best precision in quantitative measurements. Consequently, yaRAFF may offer a promising alternative for sensitization to slow molecular motion, where high resilience against off-resonance is required.
Conclusion
In simulations and phantom experiments RAFF pulses show good resilience against B1+ inhomogeneities and moderate resilience against B0 inhomogeneities. Decoupling the effective field strength from the fictitious field strength in yaRAFF helps to further improve off-resonance resilience, leading to a 40% increase in off-resonance tolerance with a maintained consistency of in-vivo precision in the calf at 3T.Acknowledgements
S.W. acknowledges funding from the 4TU Precision Medicine program, an NWO Start-up STU.019.024, and ZonMW OffRoad 04510011910073.
References
1. Mirmojarabian, S.A., et al., Myocardium Assessment by Relaxation along Fictitious Field, Extracellular Volume, Feature Tracking, and Myocardial Strain in Hypertensive Patients with Left Ventricular Hypertrophy. International Journal of Biomedical Imaging, 2022. 2022.
2. Wang, L., et al., T1rho MRI of Menisci and Cartilage in Patients with Osteoarthritis at 3T. European journal of radiology, 2012. 81(9): p. 2329-2329.
3. Liimatainen, T., et al., MRI contrast from relaxation along a fictitious field (RAFF). Magnetic Resonance in Medicine, 2010. 64(4): p. 983-994.
Figures
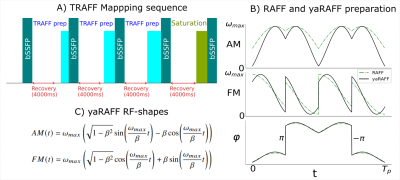
Fig1. A) Schematic representation of the TRAFF mapping sequence for four TRAFF-prepared images (0, 28, 57, 85 ms) and one saturation image with a 4s recovery in between acquisitions. Other acquisition parameters were: resolution=2x2x8 mm3, FOV=181x181x8 mm3, and the bSSFP readout with a 700 flip-angle and TE/TR=0.83/2.05ms. B) Radiofrequency shapes: amplitude modulation, frequency modulation, and phase for both RAFF and yaRAFF. C) Corresponding yaRAFF RF-shapes equation.
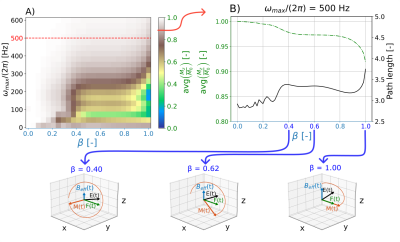
Fig 2. A) yaRAFF simulated optimization for the shape parameter, β, and peak frequency for Mz/M0 with the averaged domain (η1,Δω1)∈ [0.5,1]x[-200,200]Hz. B) Single frequency (500 Hz) yaRAFF optimization, considering the magnetization path length as a measure of spin-locking efficiency. Bottom row: Magnetization behavior at several βs, indicating the magnetization vector (M, red) with its trajectory, effective field (Beff, blue), fictitious field (F, green), and resulting field (E, black) shown in the Beff rotating frame. The optimal shape parameter was determined as β=0.62.
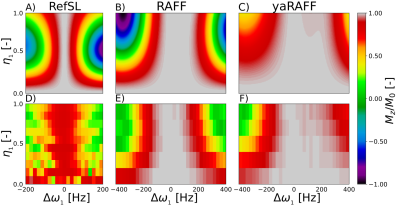
Fig 3. Performance maps for relative B0 and B+1 artifacts of Mz/M0 obtained in simulations (A, B, C) and phantom (D, E, F) for RefSL (A, D), RAFF (B, E), and yaRAFF (C, F), respectively. Here η1 is the ratio between the effective and nominal B+1 power. In simulations FWHM of 320 ± 8, 448 ± 8, and 668 ± 8 Hz, was measured for RefSL, RAFF, and yaRAFF, respectively. In phantom with a single RefSL, RAFF and yaRAFF preparation of 2.8ms and 500 Hz peak frequency FWHM of 240 ± 20, 500 ± 25 and 700 ± 50 Hz was obtained.
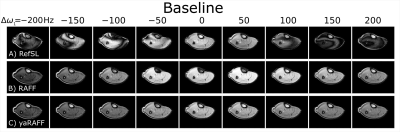
Fig 4. In vivo baseline magnitude images of the calf at nine off-resonance frequencies Δω1 = [-200, -150, …, 200] Hz Hz. baseline images of RefSL (A), RAFF (B) and yaRAFF (C) with a preparation pulse of 28 ms and peak frequency of 500 Hz (B+1,max = 13.5 μT).
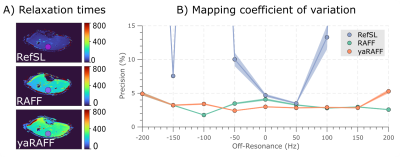
Fig 5. A) T1rho, TRAFF and TyaRAFF maps, of one healthy volunteer acquired on resonance. A circular region of interest (ROI) with muscle tissue was selected. B) The coefficient of variation of the RefSL, RAFF and yaRAFF mappings acquired in the calf of two volunteers at nine off-resonance frequencies Δω1 = [-200, -150, …, 200] Hz. The average was taken over the ROI and the two volunteers for the coefficient of variation precision measure.