2381
Variable-Density Velocity-Selective Preparation for Non-Contrast-Enhnaced MR Angiography
Minyoung Kim1,2, Jaeseok Park3,4, Seunghong Choi5, and Taehoon Shin1,2
1Department of Mechanical and Biomedical Engineering, Ewha Womans University, Seoul, Korea, Republic of, 2Graduate Program in Smart Factory, Ewha Womans University, Seoul, Korea, Republic of, 3Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of, 4Department of Intelligent Precision Healthcare Convergence, Sungkyunkwan University, Suwon, Korea, Republic of, 5Department of Radiology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea, Republic of
1Department of Mechanical and Biomedical Engineering, Ewha Womans University, Seoul, Korea, Republic of, 2Graduate Program in Smart Factory, Ewha Womans University, Seoul, Korea, Republic of, 3Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of, 4Department of Intelligent Precision Healthcare Convergence, Sungkyunkwan University, Suwon, Korea, Republic of, 5Department of Radiology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea, Republic of
Synopsis
Keywords: Pulse Sequence Design, Pulse Sequence Design, Variable Density Sampling Excitation
Velocity-selective (VS) magnetization preparation has shown great promise for non-contrast-enhanced MR angiography. Under the excitation k-space formalism, VS preparation pulse allocated RF weights to k-space at uniform intervals which leads to the aliased excitation at the inverse of the k-space interval (termed velocity FOV). In this study, we proposed a new version of VS preparation pulse with a diffused pattern of aliased saturation. The initial in-vivo test of the new version of VS-MRA shows a reduced effect of the aliased saturation compared with conventional VS-MRA.Introduction
Velocity-selective (VS) magnetization preparation has shown great promise for non-contrast-enhanced MRA. From the excitation k-space perspective, VS preparation allocates RF weights to k-space at uniform intervals, which yields notch-shaped Mz profiles with aliased saturation at the inverse of the k-space interval (termed velocity FOV) [1-4]. This aliased saturation may lead to unintended suppression of arterial blood near the velocity FOV. In this study, we propose a new version of VS preparation pulse to mitigate the issue of aliased saturation.Methods
Aliased excitation occurs at velocity FOV due to the uniform sampling of excitation k-space by VS uniformly weighted gradient pulses. We changed the sampling density function from linear to trigonometric function by re-weighting the hard RF pulses. The resultant variable density (VD) VS preparation pulse yields a diffuse pattern of aliased excitation over velocity. Under the constraint that the first moment of the entire gradient waveform is fixed to maintain the total increments of the velocity Fourier variable (kv), the trigonometric function can be parameterized by one variable which we optimized by an exhaustive search. To reduce B0/B1 sensitivity, the quadruple refocusing scheme was adapted.VD-VS and UD-VS preparations were played at the time of peak systolic flow by using ECG gating. The trigger delay (TD) of VS preparation was determined based on the prior phase-contrast flow measurements (figure 1). VD-VS preparation with trigonometric weighting function was designed with flip angle = 100°, seven hard RF sub-pulses, velocity FOV = 75 cm/s (which resulted in a cut-off velocity of 7 cm/s), and 90°-180°-90° composite pulses for quadruple refocusing within each velocity encoding step (figure 2c, f). For comparison, two versions of the uniform density (UD) VS preparation with linear weighting function were designed with the same parameters except with the velocity FOV (figure 2a, b, d, e).
The stopband [-10, 7 cm/s] of the VD-VS preparation is identical to the stopband of the UD-VS preparation with vFOV = 75 cm/s and the passband [7, 135 cm/s] of the VD-VS preparation is identical to the passband of the UD-VS preparation with vFOV = 160 cm/s. The UD-VS and VD-VS preparations were performed on healthy subjects for peripheral MRA on a clinical 3T MR scanner (Siemens Medical Solutions). Scan time = 4 min, spatial resolution = 1.0 ⅹ 1.3 ⅹ 1.6 mm3, and field of view = 360 ⅹ 194.4 ⅹ 204.8 mm3 were used. Other parameters were flip angle = 12°, TE/TR = 3.6/7.1 ms, acceleration factor = 3, view per segment = 68.
Results
Figure 3 shows representative coronal maximum-intensity-projection images of peripheral UD-VS and VD-VS MR angiography with a projection width of 10 cm. The angiogram obtained from UD-VS preparation with vFOV = 75 cm/s (figure 3a) shows a marginal signal loss in the femoral arteries presumably due to the aliased saturation. The UD-VS preparation with vFOV = 160 cm/s (figure 3b) improved the signal of the femoral arteries, but worsened the depiction of fine vessels. The VD-VS preparation with vFOV = 75 cm/s generated reasonable arterial signals in both femoral arteries and small vessels (figure 3c). The mean signal intensities of the femoral arteries were measured as, 0.52, 0.68, and 0.62 in UD-VS with vFOV = 75 cm/s, UD-VS with vFOV = 160 cm/s, and VD-VS with vFOV = 75 cm/s.Conclusion and Discussion
We have developed a variable density VS preparation pulse for non-contrast-enhanced MRA which can reduce unintended signal loss at the velocity FOV due to aliased saturation. We can identify the effect of aliased saturation by comparing UD-VS with vFOV = 75 cm/s (green arrows in figure 3a) and VD-VS with vFOV = 75cm/s (green arrows in figure 3c). We designed another UD-VS preparation with vFOV = 160 cm/s to avoid the aliased saturation. The expanded vFOV resulted in uniformly highlighted femoral arterial signal, but also suppression of the small vessels signal (yellow arrows in figure 3b). However, the signal of the small vessels in the VD-VS preparation was preserved (yellow arrows in figure 3c). The VD-VS preparation shows decreased signal loss in the femoral arteries as mitigating the aliased saturation and simultaneously maintained signal in the small vessels. Future works will include further optimization of the variable-density magnetization preparation for improving signal over the passband. A detailed comparison with the conventional uniform-density VS preparation pulses will also be conducted.Acknowledgements
This research was supported by the National Research Foundation of Korea funded by the Ministry of Education (NRF-2020R1A6A1A03043528) and the Institute of Information & communications Technology Planning & Evaluation (IITP) grant (RS-2022-00150000, Artificial Intelligence Convergence Innovation Human Resources Development (Ewha Womans University) ).References
[1] Pauly J, Nishimura DG, Macovski A. A k-space analysis of small-tipangle excitation. J Magn Reson 1989;81:43–56.
[2] de Rochefort L, MaY¨ tre X, Bittoun J, Durand E. Velocity-selective RF pulses in MRI. Magn Reson Med 2006;55:171–176.
[3] Shin T, Worters PW, Hu BS, Nishimura DG. Non-contrast-enhanced renal and abdominal MR angiography using velocity-selective inversion preparation. Magn Reson Med. 2013;69:1268-1275.
[4] Shin T, Hu BS, Nishimura DG. Off-resonance-robust velocity-selective magnetization preparation for non-contrast-enhanced peripheral MR angiography. Magn Reson Med. 2013;70:1229-1240.
Figures
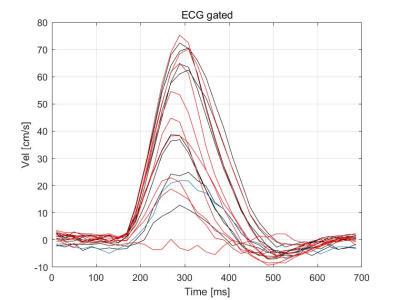
Figure
1.
Representative velocity measurement in the femoral artery. The VS preparation was
played at the time of peak systolic flow by adjusting a trigger delay (TD) based
on prior phase-contrast flow MRI.

Figure
2. (a-c)
Velocity-selective preparation pulse sequence with quadruple refocusing. (a, b)
Blue boxes show uniformly weighted gradient pulses, but (c) blue box shows variably
weighted gradient pulses. (d-f) Simulated longitudinal magnetization profile over
the velocity of on-resonance. (a, d) UD-VS with vFOV = 75 cm/s (b, e) UD-VS with
vFOV = 160 cm/s. (c, f) VD-VS with vFOV = 75 cm/s.
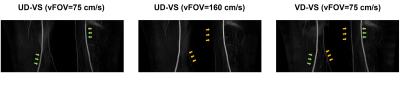
Figure
3. Coronal
MIP images of peripheral VS-MRA. (a) UD-VS with vFOV = 75 cm/s shows a signal loss
overall in the femoral arteries (green arrows). (b) UD-VS with vFOV = 160 cm/s shows
a signal loss in small vessels (yellow arrows). (c) VD-VS with vFOV = 75 cm/s shows
less signal loss in both femoral arteries and small vessels (green and yellow arrows).
DOI: https://doi.org/10.58530/2023/2381