2376
Tracking Abdominal Organ Motion in Real-time T2-weighted MRI with 2 Simultaneously Acquired Orthogonal Slices1Division of Medical Physics, Dept. of Diagnostic and Interventional Radiology, University Medical Center Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2German Cancer Consortium (DKTK), Partner Site Freiburg, Freiburg, Germany, 3ACMIT Gmbh, Wiener Neustadt, Austria, 4Division of Radiation Biophysics, Department of Radiation Oncology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States
Synopsis
Keywords: Pulse Sequence Design, Radiotherapy, Interventional, T2-weighted
Radiotherapy, cryoablation, and percutaneous needle biopsy procedures profit from MR-guidance with real-time lesion tracking. T2-weighted images often provide superior lesion contrast, but the temporal resolution of conventional T2-weighted sequences is insufficient for real-time MRI. Here, we present a fast, T2-weighted sequence Ortho-SSFP-Echo, which simultaneously acquires two orthogonal images, to track breathing induced kidney motion.
Introduction
The accuracy of therapeutic procedures such as radiotherapy, cryoablation, or percutaneous needle biopsies1,2 can be severely reduced by organ motion. Dynamic MRI is increasingly used for real-time visualization of moving targets, due to its unparalleled soft tissue contrast1. Many lesions exhibit an enhanced contrast in conventional T2-weighted MRI sequences3,4,however, these sequences are often unsuitable for real-time MRI because of their low temporal resolution. Instead, fast T1- (FLASH) or T1/T2-weighted (bSSFP) sequences are conventionally used5,6. For example, T2-weighted MRI sequences were used for pre-treatment targeting or planning of the needle trajectory in MRI-guided renal cancer cryoablation7, percutaneous renal mass biopsies8, or MR-guided radiotherapy of renal cancer9. During the procedures, a dynamic T1/T2-weighted bSSFP acquisition was applied to monitor kidney motion. In general, bSSFP images show banding artifacts which might overlap with the target region and hamper the detection. Additionally, they often only provide images of a single plane, which is insufficient to detect out-of-plane motion.Here, we present the application of a dynamic sequence designed for real-time motion monitoring in two orthogonal slices with a T2-weighted contrast, Ortho-SSFP-Echo, to track abdominal motion in an abdominal breathing phantom and in the kidney of healthy volunteers.
Methods
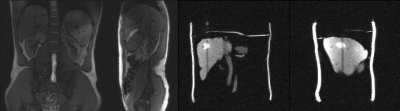
Dynamic MRI with a T2-weighted contrast can be realized with an SSFP-echo sequence that acquires the spin echo (S-) signal in the subsequent TR interval10. To acquire 2 orthogonal slices simultaneously, the sequence (Ortho-SSFP-Echo) interchanges the slice selection and phase encoding direction of 2 slices while both slices share the readout direction6, 11. Thus, the slices can be positioned such that they intersect at the lesion to monitor lesion motion. The 0th gradient moment is balanced in all gradient directions to generate a spin echo in the subsequent TR and crusher gradients ensure that signal interference between the two slices is minimized11. The SSFP-echo sequence is sensitive to motion artifacts because of the long effective TE, but this is counteracted by flow compensation in the readout direction, i.e. by balancing of the first moment.The Ortho-SSFP-Echo sequence was implemented on a clinical 3T whole body MR system (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a body array and a spine array coil for signal reception. The sequence was tested on a purpose-built abdominal breathing phantom with embedded target lesions to simulate a target-tracking scenario. Images were acquired with the following parameters: α = 30°, TR/TE = 6.78/3.38ms, BW = 1240Hz/px, SL = 6mm, FOV = 350mm2, matrix = 192, partial Fourier = 6/8, phase resolution = 80%, GRAPPA = 2, TA = 0.46s for two slices. In a post-processing step, the target lesion was contoured using the Image Segmenter App (local graph cut) in MATLAB (v. 2021b, MathWorks, MI, USA), and the lesion centroid was calculated over multiple breathing cycles.
Furthermore, the sequence was tested in a healthy volunteer to quantify kidney motion using the following acquisition parameters: α = 30°, TR/TE = 6.48/3.23ms, BW = 1300Hz/px, SL = 6mm, FOV = 400mm2, matrix = 192, partial Fourier = 6/8, phase resolution = 80%, GRAPPA = 2, TA = 0.44s for two slices. Here, the in-built MATLAB function imregdemons was used to deformably register each image within the time series to a manually contoured ground truth image. Next, the deformation field obtained from the deformable registration of each image was applied to the contour. The centroids of all contoured slices were calculated to determine the time course of the organ motion.
Results & Discussion
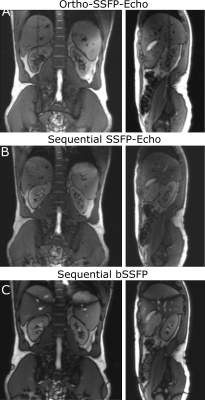
Exemplary Ortho-SSFP-Echo images of the target lesion in the abdominal phantom are show in (Fig. 2), in which the target lesion mask is overlaid onto the orthogonal tracking images. In the images, the position of the other orthogonal slice can be identified as a saturation band. The range of lesion motion is shown in Figure. 2c as a function of time.In the volunteer experiments, breathing-induced motion of the kidney was observed (Fig. 3). Figure 4 provides an in vivo comparison of the Ortho-SSFP-Echo sequence, a reference single slice SSFP-echo sequence, and a bSSFP sequence. Banding artifacts are visible in the bSSFP image. The total range of motion in the coronal slice was 4.2mm / 6.8mm in right-left / head-foot direction, and 7.2mm / 7.0mm in the anterior-posterior / head-foot direction in the sagittal slice.
In the current implementation, 2 orthogonal slices were acquired in 0.46s (frame rate: 2Hz), which is sufficient for resolving breathing motion in needle guidance and cryoablation applications. Lowering the temporal resolution could allow for radiotherapy or cardiac applications. Motion-induced dephasing could be partially compensated by flow compensation; however, this requires a careful choice of the readout direction.
MR-guided cryoablation, percutaneous needle biopsies, and radiotherapy of renal cancer would all benefit from real-time targeting/tracking T2-weighted sequences. Ortho-SSFP-Echo offers the desired T2-weighted contrast, as well as a truly simultaneous acquisition, allowing detection of in- and out-of-plane motion.
Conclusion
Application of the Ortho-SSFP-Echo sequence in the abdomen allowed kidney motion to be effectively observed simultaneously in two orthogonal T2-weighted slices. In a post-processing step, the breathing induced motion was successfully tracked and measured. Future applications of the Ortho-SSFP-Echo sequence could include MR-guidance in percutaneous needle interventions, cryoablation, or lesion tracking in radiotherapy.Acknowledgements
This work was supported in part by a grant from the German Federal Ministry for Economic Affairs and Energy (BMWi) under the grant program “Zentrales Innovationsprogramm Mittelstand (ZIM),” grant number ZF4535603BA9, as part of the IraSME funding “E‐GantryMate”.
References
1. Kurz C, et al. Medical physics challenges in clinical MR-guided radiotherapy. Radiat Oncol. 2020;15(93).
2. Pham D, et al. A review of kidney motion under free, deep and forced-shallow breathing conditions: implications for stereotactic ablative body radiotherapy treatment. Technol Cancer Res Treat. 2014;13(4):315-323.
3. Chetty IJ, et al. Technology for Innovation in Radiation Oncology. Int J Radiat Oncol Biol Phys. 2015;93(3):485-492.
4. Jansen EP, et al. Target volumes in radiotherapy for high-grade malignant glioma of the brain. Radiother Oncol. 2000;56(2):151-156.
5. Tryggestad E, et al. 4D tumor centroid tracking using orthogonal 2D dynamic MRI: implications for radiotherapy planning. Med Phys. 2013;40(9):091712.
6. Mickevicius NJ, et al. Simultaneous orthogonal plane imaging. Magn Reson Med. 2017;78(5):1700-1710.
7. De Marini P, et al. Safety and oncologic efficacy of percutaneous MRI-guided cryoablation of intraparenchymal renal cancers. Diagn Interv Imaging 2021;102(9):531-538.
8. Garnon J, et al. Evaluation of percutaneous biopsies of renal masses under MRI-guidance: a retrospective study about 26 cases. Eur Radiol 2015;25(3):617-623.
9. Keller B, et al. Adaptive Magnetic Resonance-Guided Stereotactic Body Radiotherapy: The Next Step in the Treatment of Renal Cell Carcinoma. Front Oncol 2021;11:634830.
10. Gyngell ML. The application of steady-state free precession in rapid 2DFT NMR imaging: FAST and CE-FAST sequences. Magn Reson Imaging. 1988;6(4):415-419.
11. Krafft AJ, et al. Crushed rephased orthogonal slice selection (CROSS) for simultaneous acquisition of two orthogonal proton resonance frequency temperature maps. J Magn Reson Imag 2013:38(6): 1510-1520.
Figures
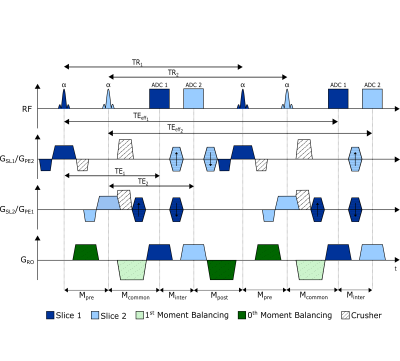
Ortho-SSFP-Echo pulse sequence diagram. The slice selection and phase encoding directions are interchanged: the slice selection direction of slice 1 (GSL1) is the phase encoding direction of slice 2 (GPE2) and vice versa. Slice 1 and slice 2 elements of the sequence are shaded dark blue and light blue, respectively. The gradients added to balance the zeroth and first moments in the readout direction are shaded in dark and light green, respectively.
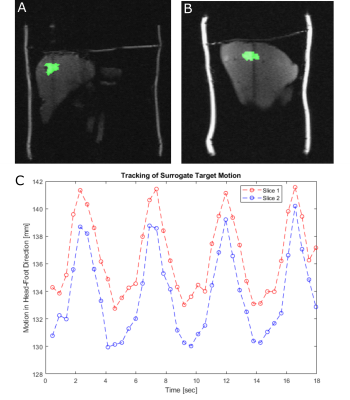
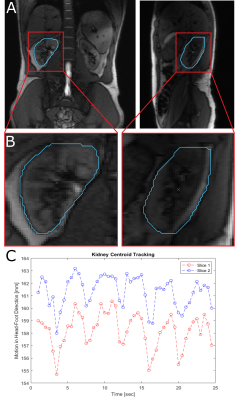
(A) In vivo orthogonal images (coronal and sagittal) of the kidney, acquired with the Ortho-SSFP-Echo sequence. (B) The contoured right kidney is illustrated alongside its corresponding centroid. (C) The kidney displacement, in the head-foot direction, due to breathing induced motion is shown for each orthogonal slice.