2371
Synthetic MRI in differentiating benign from metastatic retropharyngeal lymph nodes: combination with diffusion-weighted imaging1Affiliated hospital of Jiangnan university, Wuxi, China, 2ffiliated hospital of Jiangnan university, Wuxi, China, 3GE Healthcare, MR Research China, Beijing, China
Synopsis
Keywords: Head & Neck/ENT, Quantitative Imaging
This study aimed to evaluate MAGiC imaging (one synthetic MRI technique, syMRI) and its combination with diffusion-weighted imaging (DWI) in discriminating benign from metastatic retropharyngeal lymph nodes (RLNs). With MAGiC derived relaxation parameters, 58 patients with 21 benign and 42 metastatic RLNs were measured. The resultant T1, T2, PD and T1SD values showed significant different values between benign and metastatic RLNs with an optimal diagnostic performance from T1SD. Moreover, the combination of MAGiC, DWI, and morphological features demonstrated a significantly improved performance on overall diagnosis.Introduction
Precise treatment planning and satisfactory prognosis of patients are determined, to a great extent, by an accurate detection of retropharyngeal lymph node (RLN) metastases in nasopharyngeal carcinoma [1-3]. Magnetic resonance diffusion weighted imaging (DWI) is often used for clinical diagnosis due to its ability to quantitatively analyze the physiological characteristics of tissues and help distinguish benign from malignant RLNs [4]. However, its inconsistent performance in some cases may lead to unnecessary or inadequate radiation treatment [5-8]. Magnetic resonance image compilation (MAGiC), as one type of synthetic MRI (syMRI), is a relatively novel quantitative MRI technique that can offer a comprehensive set of relaxometry mapping of T1, T2 and PD in a single measurement [9]. However, it has rarely been reported to evaluate the diagnostic performance in distinguishing benign from metastatic RLNs so far. Therefore, the main goal of this work was to explore the clinical feasibility of MAGiC imaging in differentiating the characteristics of benign from metastatic RLNs by applying two different ROI delineation methods, and further investigate the corresponding diagnostic efficacy of combined MAGiC, DWI and morphological features.Method and Materials
SubjectsA total of 58 patients with 21 benign RLNs and 42 metastatic RLNs were include in this study. Ethical approval and consent forms were obtained.
MRI experiments
All patients underwent MR scans on a 3.0T scanner (Signa Architect, GE, USA) with a 28-channel phased array coil. MAGiC imaging was added to a routine MRI protocol prior to contrast injection, with scan parameters as follows: repetition time=4000 ms, echo time=14.2/92.1 ms, echo train length=16, field-of-view=24×24 cm2, slice thickness/gap=4/0.4 mm, matrix=320×256, NEX=1. Mean and standard deviation of MAGiC derived parameters (T1, T2, PD; T1SD, T2SD, PDSD) were obtained. Single-shot DWI was also acquired by setting parameters as: repetition time=3457 ms, echo time=1.0 ms, matrix=128 × 130, field-of-view=24×24 cm2, and slice thickness/gap=4.0/1.0 mm. Two gradient factors (b = 0, 1000 s/mm2) were used to determine the apparent diffusion coefficient (ADC) map with mono-exponential fitting.
Data analysis
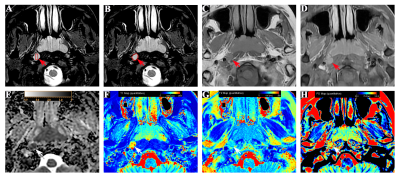
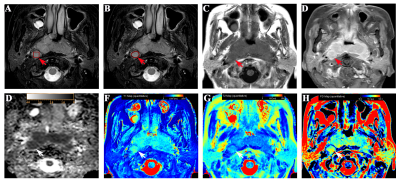
Two neuroradiologists independently delineated ROIs by two methods (partial-lesion, full-lesion) based on T1WI and T2WI. The morphological features (size, signal homogeneity, T2WI signal intensity, and border) were evaluated in each RLNs. The ROIs with the largest area of the largest section were also copied on ADC maps, and mean ADC levels were obtained accordingly. The student’s t-test for non-categorical data and chi-square tests for categorical variables were used to carry out the comparisons among parameters derived from MAGiC imaging and DWI. Receiver operating characteristic (ROC) curve and areas under the curve (AUC) analysis were used to analyze the diagnostic efficiency for each parameter. The logistic regression analysis was operated to construct a multi-parameter diagnostic model of MAGiC, MAGiC+DWI, and MAGiC+DWI+morphological features, using Delong test to determine the best diagnostic approach. Pearson correlation coefficient was used to evaluate the correlation between each of MAGiC parameters and ADC. All the above analyses were performed by using MedCalc software (version 15.2.2) and SPSS software (version 22.0). P<0.05 was considered statistically significant.
Results
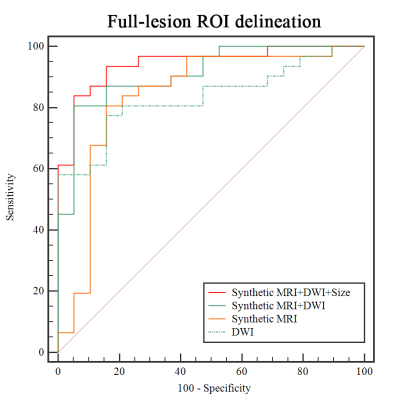
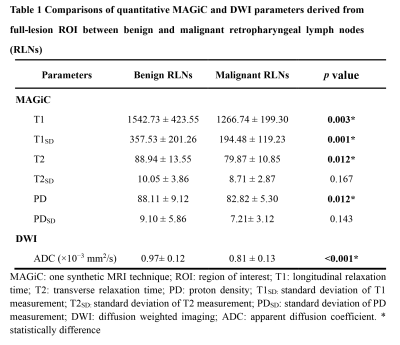
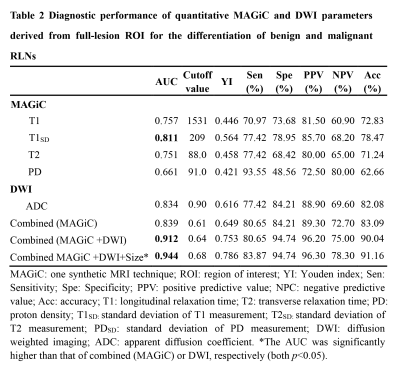
MAGiC derived parameters (T1, T2, PD, T1SD) were significantly higher in benign than malignant RLNs for both partial-lesion and full-lesion ROI delineation methods (all p<0.05). Similar results were also found for DWI derived quantitative parameter (ADC; p<0.05). No significant difference was found in T2SD and PDSD between benign and metastatic RLNs, regardless of the ROI delineation method (both p > 0.05, Fig.1-2, Table 1). In Table 2, T1SD values obtained from full-lesion ROI delineation showed the best diagnostic efficacy for distinguishing benign from metastatic RLNs with the highest AUC (0.811) among all MAGiC derived single parameters. Moreover, the combination of MAGiC and DWI derived significant parameters (T1SD, PD, ADC) as well as the morphological feature of size can significantly improve the AUC value to 0.944 (Fig. 3).Discussion
In this study, we investigated the clinical utility of MAGiC imaging (a synthetic MRI technique) derived quantitative mapping and its combination with DWI in distinguishing benign from metastatic RLNs. The resultant T1, T2, PD, T1SD and ADC values have been demonstrated higher in benign than malignant RLNs for both partial-lesion and full-lesion ROI delineation methods. The possible explanation might be that higher cellularity and nuclear-to-cytoplasmic ratios in malignant lesions restrict the free diffusion of water molecules, which may lead to a corresponding decrease in the extracellular fluid space and free water content [10-13], resulting lower T1, T2, PD and ADC. For lower T1SD in metastatic RLNs, it may be caused by dense arrangement of tumor cells, which may have less T1 variation within ROIs. Moreover, the combination of MAGiC imaging and DWI derived quantitative parameters as well as morphological feature of size was validated with significantly improved diagnostic efficiency that in differentiating these two entities by the highest AUC. This finding might be meaningful for preoperative determination of radiation treatment, which might reduce the number of unnecessary or inadequate radiation treatment.Conclusion
In conclusion, the clinical value of MAGiC imaging has been validated in differential diagnosis of benign and metastatic RLNs, and the combined MAGiC, DWI and morphological features can further improve the diagnostic efficiency for discriminating these two entities.Acknowledgements
No acknowledgement found.References
1 Huang L, Zhang Y, Liu Y et al (2019) Prognostic value of retropharyngeal lymph node metastasis laterality in nasopharyngeal carcinoma and a proposed modification to the UICC/AJCC N staging system. Radiotherapy and Oncology 140:90-97
2 Lee AW, Ng WT, Pan JJ et al (2018) International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiotherapy and Oncology 126:25-36
3 Lee AW, Ma BB, Ng WT, Chan AT (2015) Management of Nasopharyngeal Carcinoma: Current Practice and Future Perspective. Journal of Clinical Oncology 33:3356-3364
4 Abdel RA, Soliman NY, Elkhamary S, Alsharaway MK, Tawfik A (2006) Role of diffusion-weighted MR imaging in cervical lymphadenopathy. European Radiology 16:1468-1477
5 Jin GQ, Yang J, Liu LD et al (2016) The diagnostic value of 1.5-T diffusion-weighted MR imaging in detecting 5 to 10 mm metastatic cervical lymph nodes of nasopharyngeal carcinoma. Medicine (Baltimore) 95:e4286
6 Chen C, Lin Z, Xiao Y et al (2018) Role of diffusion-weighted imaging in the discrimination of benign and metastatic parotid area lymph nodes in patients with nasopharyngeal carcinoma. Sci Rep 8:281
7 Li H, Liu XW, Geng ZJ, Wang DL, Xie CM (2015) Diffusion-weighted imaging to differentiate metastatic from non-metastatic retropharyngeal lymph nodes in nasopharyngeal carcinoma. Dentomaxillofac Radiol 44:20140126
8 Pekcevik Y, Cukurova I, Arslan IB (2015) Apparent diffusion coefficient for discriminating metastatic lymph nodes in patients with squamous cell carcinoma of the head and neck. Diagnostic and Interventional Radiology 21:397-402
9 Warntjes JB, Leinhard OD, West J, Lundberg P (2008). Rapid magnetic resonance quantification on the brain: Optimization for clinical usage. Magnetic Resonance in Medicine. 2008 Aug;60(2):320-329.
10 Jung Y, Gho SM, Back SN, Ha T, Kang DK, Kim TH (2018) The feasibility of synthetic MRI in breast cancer patients: comparison of T2 relaxation time with multiecho spin echo T2 mapping method. Br J Radiol:20180479
11 Cai Q, Wen Z, Huang Y et al (2021) Investigation of Synthetic Magnetic Resonance Imaging Applied in the Evaluation of the Tumor Grade of Bladder Cancer. Journal of Magnetic Resonance Imaging 54:1989-1997
12 Connolly M, Srinivasan A (2018) Diffusion-Weighted Imaging in Head and Neck Cancer: Technique, Limitations, and Applications. Magn Reson Imaging Clin N Am 26:121-133 13 Payabvash S (2018) Quantitative diffusion magnetic resonance imaging in head and neck tumors. Quant Imaging Med Surg 8:1052-1065
Figures