2366
Highly efficient T1, T2 and Diffusion-prepared radial Magnetic Resonance Fingerprinting
Carlos Velasco1, Carlos Castillo-Passi1,2, Nadia Chaher1, Alkystis Phinikaridou1, René M. Botnar1,2, and Claudia Prieto1,2
1School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 2Institute for Biological and Medical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile
1School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 2Institute for Biological and Medical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile
Synopsis
Keywords: MR Fingerprinting/Synthetic MR, Diffusion/other diffusion imaging techniques
In this study we present a golden-angle-radial efficient magnetic resonance fingerprinting approach that enables simultaneous T1, T2 and ADC quantification in a single scan of ~18s and offers the possibility to be extended to a multi-echo acquisition for additional water-fat separation estimation. A quantitative comparison between reference maps and the proposed MRF maps has shown excellent agreement, and a proof-of-concept brain MRF multiparametric T1, T2 and ADC has shown comparable image quality compared to the longer and sequential clinical reference scans.Introduction
Multiparametric MRI has gained interest in recent years as it may provide a more comprehensive understanding of the underlying pathologies1,2. In addition, certain confounding factors may be eliminated if they are estimated simultaneously during the same multiparametric acquisition. Multiparametric studies of T1, T2 and apparent diffusion coefficient (ADC) have been traditionally performed in sequential scans3, which are prone to mis-registration artifacts. Recently, two Magnetic Resonance Fingerprinting (MRF) approaches with spiral readout have been proposed for simultaneous generation of T1, T2 and ADC maps of the brain4,5. In this study we propose an alternative radial acquisition with golden-angle increment approach that enables simultaneous T1, T2 and ADC quantification in a single scan of ~18s and offers the possibility to be extended to a multi-echo acquisition for additional water-fat separation estimation.Methods
A gradient echo MRF sequence with golden radial trajectory and varying magnetization preparation pulses before each acquisition block is proposed (Figure 1a). This MRF acquisition consists of 18 acquisition blocks preceded by one of the following magnetization preparation pulses: two inversion recovery (IR) pulses (TIs = {10,150}ms), four T2 preparation pulses (T2ps = {35,60}ms), eight diffusion preparation pulses (b-values = {200,400,600,800} s/mm2) and four additional blocks in which no magnetization preparation is applied to allow for magnetization recovery. In particular, the diffusion preparation had a total duration of 55ms, including two adiabatic refocusing pulses of 10.3 ms inserted in-between the diffusion gradients to increase robustness against field inhomogeneities. The diffusion pulse was optimized to reach the highest possible b-value for a given diffusion preparation time and considering technical limitations6 (including concomitant gradients effect, zeroth moment nulling and hardware max. gradient and slew-rate specifications). Each acquisition block contains a train of 30 shots with sinusoidally varying flip angles in the range 10°-20°. Echo and repetition times are TE = 3.3ms and TR = 7.0ms. Reconstruction is performed using a wavelet-regularized sparse and locally low-rank approach. Other acquisition parameters of interest are: 2x2mm2 resolution, 8mm slice thickness and acquisition time ~18s.The proposed approach was evaluated in phantoms: a) a conventional T1MES for T1, T2 validation and b) a set of tubes filled with a fixed concentration of NiCl2 and Agarose to obtain physiological T1, T2 values, as well as a varying concentrations of polyvinylpyrrolidone (PVP) to generate a range of isotropic diffusion values7. The sequence was additionally tested in a healthy volunteer. All scans were performed on a 3T scanner (Achieva TX, Philips Healthcare) and compared against conventional individual single breath hold maps (T1-MOLLI, T2-GRaSE, and DTI-SE, for T1, T2 and ADC estimation respectively). The diffusion preparation pulse of the MRF scans was applied along a fixed Cartesian direction throughout the whole scan. Diffusion was considered isotropic within the phantom tubes.
Results
A qualitative comparison between the maps obtained with the proposed MRF technique and reference maps is shown in Figure 2 for the two phantoms evaluated. Scan times of reference scans were: 12s, 18s and 123s for T1-MOLLI, T2-GRaSE and DTI-SE, respectively. T1-MRF, T2-MRF and ADC-MRF maps were obtained from the same single acquisition of 18s. A quantitative comparison between reference maps and the proposed MRF maps is shown in Figure 3, where an excellent agreement is shown for all three measurements, with Pearson coefficient values of r2 = 0.997, r2 = 0.95 and r2 = 0.96 for T1, T2 and ADC respectively.Figure 4 depicts a proof-of-concept acquisition in a healthy subject that shows a qualitative comparison of T1, T2 and diffusion maps obtained in the volunteer’s brain in axial orientation (Figure 4a: reference T1-MOLLI, T2-GRaSE and DTI-SE maps; Figure 4b: T1-MRF, T2-MRF and ADC-MRF).
Discussion
In this work, we propose an IR, T2 and Diffusion prepared MRF sequence for T1, T2 and diffusion mapping of brain tissue in a single scan of ~18s with golden angle radial trajectory. The proposed MRF sequence has been demonstrated to generate simultaneous T1, T2 and diffusion maps in the brain of a healthy volunteer. Moreover, qualitative, and quantitative phantom evaluation has shown the ability of the proposed sequence to measure ADC in the range of 1.0 – 2.0 mm2/s, with T1 and T2 values being in the physiological range (T1≈900ms and T2≈45ms). Nevertheless, accurate quantification of diffusion remains challenging for tissues with lower T2 due to the rapid bi-exponential signal decay (diffusion and T2) that takes place during the diffusion preparation pulse. Motion during the diffusion pulse also can lead to signal loss further adding to this challenge. Further optimization is warranted to achieve higher moment-nulling of the diffusion preparation waveform to increase robustness against bulk motion and allow for ADC quantification within organs that present non-negligible motion such as the liver or the heart. Additionally, stronger diffusion preparation gradients and shorter preparation times of the diffusion preparation pulse will be explored, achieved by replacing of the two adiabatic hyperbolic secant pulses for one BIR-4. In addition, the radial trajectory employed can be extended with multi-echo readouts thereby enabling water-fat estimation and a more comprehensive quantification of tissue parameters in organs where the presence of fat is non-negligible, such as the liver.Acknowledgements
This work was supported by the following grants: (1) EPSRC P/V044087/1,(2) BHF programme grant RG/20/1/34802, (3) Wellcome/EPSRC Centre for Medical Engineering (WT 203148/Z/16/Z), (4) Millennium Institute for Intelligent Healthcare Engineering ICN2021_004, (5) FONDECYT 121074, 1210637 and 1210638, (6) IMPACT, Center of Interventional Medicine for Precision and Advanced Cellular Therapy, ANID FB210024, (7) PhD program in Biological and Medical Engineering of the Pontificia Universidad Católica de Chile.References
1Kim, P.K. et al., Korean J. Radiol. 2017.
2Messroghli, D.R. et al., JCMR 2017.
3Yu, A.C. et al., Radiology 2017.
4Cao, X. et al. ISMRM2022 Abs #0101.
5Afzali, M. et al., MRM 2022.
6Peña-Nogales, O., et al, MRM 2019.
7Wagner, F. et al., PLoS ONE 2016.
Figures
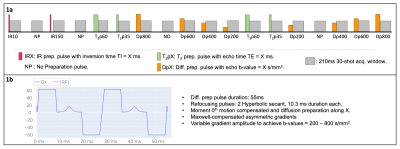
Figure 1. A) Schematic drawing of sequence proposed. Red, green and orange blocks depict T1, T2 and diffusion preparation pulses applied before each acquisition block (grey boxes). A more detailed description of the diffusion preparation pulse is shown in b). Diffusion preparation gradients are applied along a fixed cartesian direction, in a total duration of 55ms. Two refocusing sech adiabatic pulses, of duration 10.3 ms are included in this preparation to make it robust against field inhomogeneities. Maximum gradient applied is tuned to achieve b-vales in the range 200-800 s/mm2.
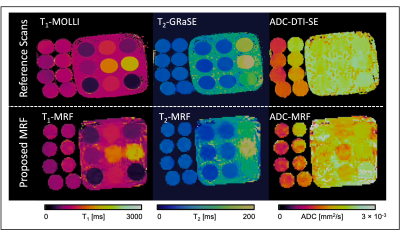
Figure 2. Top: T1, T2 and ADC map obtained from T1-MOLLI, T2-GRaSE and DTI-SE-prepared scans respectively. Bottom: Corresponding T1, T2 and ADC maps obtained simultaneously from the same MRF sequence, showed in Fig. 1a. The phantom of the left is comprised of eight tubes with fixed NiCl2 and agarose concentration to achieve a fixed T1 and T2 values, plus a variable concentration of PVP to achieve different ADC values. The phantom of the right is the commercial T1MES, with different concentrations of NiCl2 and agarose to achieve T1 and T2 values, also in physiological range.
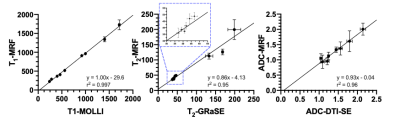
Figure 3. Pearson correlation plots of values measured from ROIs manually drawn within the phantoms shown in Fig. 2. T1, T2 and ADC quantification obtained from the MRF scan is compared against their respective references. 9 circular ROIs were manually drawn within T1MES phantom for T1 and T2 comparison, whereas 8 circular ROIs from the custom-made diffusion phantom were employed for ADC evaluation. Inset shown in middle graph (T2 evaluation) shows a zoomed area of the datapoints surrounded with a blue box.
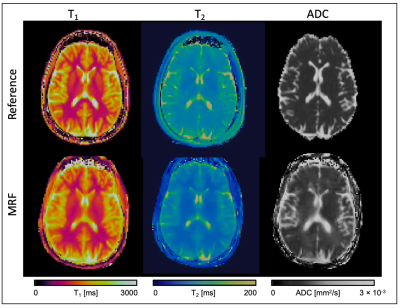
Figure 4. Proof of concept of parametric maps of T1 (left), T2 (middle) and Diffusion measured along X direction (right) of a healthy subject that underwent the proposed 18s radial MRF acquisition (bottom) and the corresponding reference T1-MOLLI, T2-GRaSE and DTI-SE scans.
DOI: https://doi.org/10.58530/2023/2366