2364
Efficient MRF at 0.55T with long readouts and concomitant field effects correction1Department Of Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 2Department Of Biomedical Engineering, University of Southern California, Los Angeles, CA, United States
Synopsis
Keywords: MR Fingerprinting/Synthetic MR, Brain
We demonstrate FISP-MRF at 0.55T with improved efficiency using longer spiral readouts and concomitant field effect correction. 2D axial FISP-MRF is performed with 3 different readout durations and at 2 different off-isocenter locations, reconstructed without and with MaxGIRF concomitant field correction. Spatial blurring induced by concomitant field effect was successfully mitigated. In-vivo MRF white matter T2 achieved tighter distribution with longer readouts, e.g., the coefficient of variation decreased from 9.9% to 4.9%.Introduction
FISP-MRF1,2 has been extensively studied at 1.5T and 3T and has been recently demonstrated with good repeatability at 0.55T3,4 and even lower field strengths5. However, the benefits of low-field MRF have not yet been fully explored. For example, improved field homogeneity enables longer readouts, and higher scan efficiency, which partially compensates for the reduced SNR due to equilibrium polarization (proportional to B0). When doing so, the concomitant field effect which is stronger at low field strengths and when using stronger gradients, requires mitigation. In this study, we evaluate 2D axial FISP-MRF with 3 readout durations and 2 reconstruction approaches at 0.55T.Methods
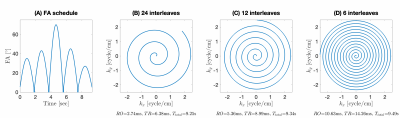
Experiments were performed using a whole body 0.55T system (prototype MAGNETOM Aera,Siemens Healthineers, Erlangen, Germany) equipped with high-performance shielded gradients (45 mT/m amplitude, 200 T/m/s slew rate). The 2D FISP-MRF sequences with spiral readouts (RO) were developed using SpinBench and executed using the RTHawk console (HeartVista, Inc., Menlo Park, CA). Acquisition details are shown in Figure 1. All 2D MRF scans were prescribed in the axial orientation and were performed at isocenter and 50mm off-isocenter locations, e.g., $$$\Delta z$$$=0 and $$$\Delta z$$$=50mm, using different RO to induce a different amount of through-plane concomitant field effect.Two image reconstruction approaches were compared: (a) SENSE and (b) Maxwell Gradient Impulse Response Function (MaxGIRF)6 both with low-rank and subspace modeling7. Dictionaries corresponding to different MRF schedules were generated using the slice-selective extended phase graph (ssEPG)8 approach for the subspace modeling and dictionary matching process. Static off-resonance maps $$$\Delta f$$$ required by MaxGIRF were estimated from multi-GRE data with matching FOV and resolution as MRF.
Evaluations were performed in the ISMRM/NIST system phantom9 and in 1 healthy volunteer (34M). For both phantom and volunteer scans, MRF results were compared among 3 RO, 2 slice positions and 2 reconstruction approaches.
Results
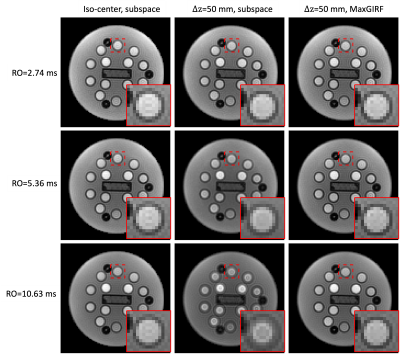
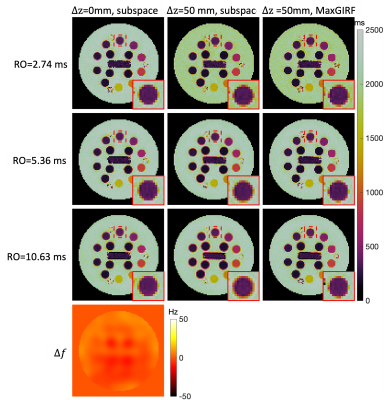
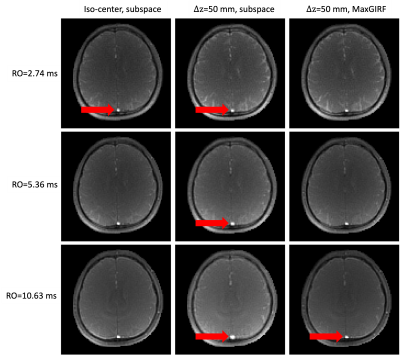
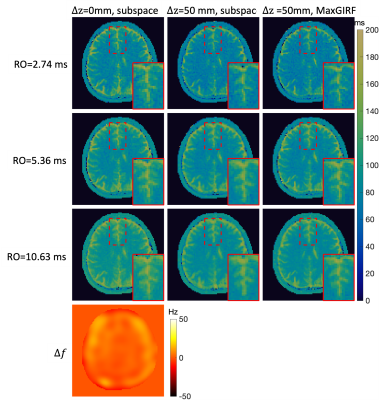
Phantom MRF: Figure 2 shows representative MRF images at FA=70°. Images of RO durations from short to long are shown in row 1 to 3. $$$\Delta f$$$ map is shown in row 4. Images reconstructed by approach (a) show more severe blurring when a longer RO is used and the distance from isocenter increases, as shown in column 1 and 2. In column 3, MRF images corrected by approach (b) are shown. Zoomed-in views of an example are shown in the right bottom of each subfigure. Figure 3 shows the corresponding MRF T1 maps of images in Figure 2. Slight blurring on vial boundaries in T1 maps is observed. Matched zoomed-in views as in Figure 3 are shown.In-vivo MRF: Figure 4 shows representative MRF images at FA=70°. Severe blurring was observed as expected at a longer RO duration and a larger distance from isocenter. An example of blurring and its correction around the superior sagittal sinus is pointed out by red arrows. Figure 5 shows the corresponding MRF T2 maps. Enhanced blurring on white matter and grey matter boundaries was observed in column 2, especially in the long RO case. Such blurring was well mitigated by approach (b). Zoomed-in views are shown in the right bottom of each subfigure. T2 values in white matter were more homogeneous and had a tighter distribution for longer RO. Quantitatively, T2 coefficient of variation (COV) in white matter measured in this study were 9.9%, 7.3% and 4.9% from short to long RO when $$$\Delta z$$$=0mm, and are 10.1%, 7.1% and 5.4% after MaxGIRF correction when $$$\Delta z$$$=50mm.
Discussion
We demonstrate 2D FISP-MRF at 0.55T with substantially longer RO duration compared to what is typically used at 1.5T and 3T and demonstrate a reconstruction method to simultaneously mitigate static off-resonance and concomitant field effect. The results clearly show the dependencies of concomitant field effect on RO duration and distance from isocenter, both in the phantom and in-vivo data. At longer RO and larger distance, the concomitant field induced blurring became worse, however, better SNR was achieved with the same spatial resolution. With correction, high-quality MRF results with clear boundaries and improved homogeneity were achieved given the limited scan time, as shown in Figure 5.There are several limitations of this study. We considered only concomitant fields induced phase accumulation within RO duration, while phase accumulation through multiple TRs was ignored. We expect that this can be addressed by utilizing unique dictionaries for each voxel, incorporating concomitant field effect and/or field inhomogeneity. This is of interest for future work.
This study only considered 2D axial imaging with moderate resolution for practical purposes. As a result, this design simplifies the concomitant field effect, such that it was only dependent on $$$\Delta z$$$ given the same RO gradients but was independent of voxels’ in-plane locations. It can be generalized to 3D imaging with stack of spirals, where z-gradients and RO gradients are not simultaneously used. Thus, z-gradients induce additional effect dependent on in-plane locations only and can be combined with in-plane effect. However, the concomitant effect would be more complicated when using prescriptions such as sagittal or oblique, or 3D trajectories such as maSPI10 or 3D cones11.
Conclusion
We demonstrate FISP-MRF at 0.55T with longer RO durations and MaxGIRF concomitant field effect correction. Deblurring was effective. This approach provides improved MRF scan efficiency.Acknowledgements
We acknowledge grant support from the National Science Foundation (#1828736) and research support from Siemens Healthineers. We are grateful to MREL/DISC members for helpful discussion.References
1. Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magn Reson Med. 2015;74(6):1621-1631. doi:10.1002/mrm.255592.
2. Ma D, Jiang Y, Chen Y, et al. Fast 3D Magnetic Resonance Fingerprinting for a Whole-Brain Coverage. Magn Reson Med. 2018;79:2190-2197. doi:10.1002/mrm.268863.
3. Campbell-Washburn AE, Jiang Y, Körzdörfer G, Nittka M, Griswold MA. Feasibility of MR fingerprinting using a high-performance 0.55 T MRI system. Magn Reson Imaging. Published online June 8, 2021. doi:10.1016/j.mri.2021.06.0024.
4. Zhu Z, Lee NG, Tian Y, et al. Evaluation of MR Fingerprinting at 0.55T. In: ISMRM 31st Scientific Session. ; 2022:3492. Accessed November 6, 2022. https://cds.ismrm.org/protected/22MPresentations/abstracts/3492.html5.
5. Sarracanie M. Fast Quantitative Low-Field Magnetic Resonance Imaging With OPTIMUM-Optimized Magnetic Resonance Fingerprinting Using a Stationary Steady-State Cartesian Approach and Accelerated Acquisition Schedules. Invest Radiol. 2022;57(4):263-271. doi:10.1097/RLI.00000000000008366.
6. Lee NG, Ramasawmy R, Lim Y, Campbell-Washburn AE, Nayak KS. MaxGIRF: Image reconstruction incorporating concomitant field and gradient impulse response function effects. Magn Reson Med. 2022;88(2):691-710. doi:10.1002/MRM.292327.
7. Zhao B, Setsompop K, Adalsteinsson E, et al. Improved magnetic resonance fingerprinting reconstruction with low-rank and subspace modeling. Magn Reson Med. 2018;79(2):933-942. doi:10.1002/mrm.267018.
8. Ostenson J, Smith DS, Does MD, Damon BM. Slice-selective extended phase graphs in gradient-crushed, transient-state free precession sequences: an application to magnetic resonance fingerprinting. Magn Reson Med. 2020;84(6):3409. doi:10.1002/MRM.283819.
9. Stupic KF, Ainslie M, Boss MA, et al. A standard system phantom for magnetic resonance imaging. Magn Reson Med. 2021;86(3):1194-1211. doi:10.1002/mrm.2877910.
10. Cao X, Ye H, Liao C, Li Q, He H, Zhong J. Fast 3D brain MR fingerprinting based on multi-axis spiral projection trajectory. Magn Reson Med. 2019;82(1):289-301. doi:10.1002/MRM.2772611.
11. Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magn Reson Med. 2006;55(3):575-582. doi:10.1002/MRM.20796.
Figures