2355
Role of MULTIPLEX MRI Study in Evaluating Brain Tumors1Department of Radiology, The Second Xiangya Hospital of Central South University, Changsha, China, 2Shenzhen United Imaging Research Institute of Innovative Medical Equipment, Shenzhen, China, 3MR Collaboration, Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
Keywords: MR Fingerprinting/Synthetic MR, MR Fingerprinting
Multi-parametric MR imaging methods have been intensively developed and investigated throughout the last decade. The purpose of this study was to utilize the single-scan 3D multi-parametric MRI technique, MULTIPLEX, to characterize and grade intracranial tumors. According to the results, MULTIPLEX MRI can effectively decrease the scan time while obtaining multi-parametric images and mappings. Besides, the simultaneous quantitative estimation of multiple MR parameters can reliably characterize and grade intracranial tumors.Introduction
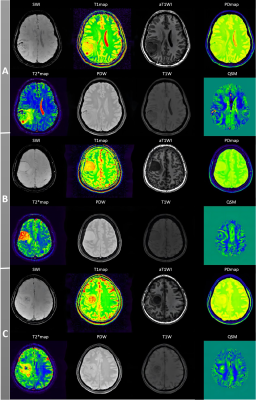
Magnetic resonance imaging (MRI) is now widely used to assess intracranial tumors [1, 2]. However, practical and technical challenges have limited the use of MRI in daily practice. For one thing, relatively long scanning times can be an obstacle, especially for young children and patients with severe diseases. For another, signal intensities on conventional MR images are affected by the sequences utilized, varying coil sensitivities, and B1 field inhomogeneities [3]. Multi-parametric MR imaging methods have been intensively developed and investigated throughout the last decade. The GRE sequence has the potential to be utilized as the basis for developing multi-parametric approaches. GRE acquisition using a signal spoiling mechanism to collect incoherent steady state (ISS) signals may have certain advantages, including 3D high-resolution (eg, voxel size <1 mm3), imaging capacity [4], high data acquisition efficiency, well-formulated signal models, as well as compatibility with various sequence design [5] and acceleration techniques [6]. Recently, a gradient echo (GRE)-based method, namely multi-parametric MRI with flexible design (MULTIPLEX), is proposed to acquire 3D high-resolution imaging [7]. The MULTIPLEX sequence features a dual-repetition time (TR), dual-flip angle (FA), and multi-echo design. One single MULTIPLEX scan enables simultaneous generation of the B1 field, qualitative images, including T1-weighted (T1W), proton density-weighted (PDW), T2*-weighted (T2*W), augmented T1W (aT1W), and susceptibility weighted imaging (SWI), as well as parametric maps, including T2* map, R2* map, T1 map, PD map, and quantitative susceptibility mapping (QSM) in only about 7 minutes for full-head coverage. This study aimed to utilize the single-scan 3D multi-parametric MRI technique, MULTIPLEX, to generate multiple parametric maps at the same time, and to characterize and grade intracranial tumors by these parameters.Methods
The patients with intracranial space-occupying lesions were included in this prospective study before the operation. For each patient, MULTIPLEX was performed on a 3T MR scanner (uMR790, United Imaging Healthcare, Shanghai, China). The key scanning parameters for MULTIPLEX were: TR1/TR2 = 8.8 ms/26.3 ms, FA1/FA2 = 4°/16°, five echoes with TE = 3.46 ms, 8.6 ms, 12.03 ms, 17.17 ms, and 20.6 ms, Bandwidth = 200 Hz/px, field-of-view = 190 × 224 mm2, matrix size = 190 × 320, slice thickness = 2 mm. The total acquisition time was 7 minutes and 17 seconds. For all the quantitative maps, two regions of interest (ROIs) within the intratumoral and peritumoral volumes were evaluated by two experienced radiologists. The Mann-Whitney test was used to compare quantitative parameters between glioma and meningioma, as well as low-grade and high-grade glioma. The receiver operating characteristic analysis was performed to assess the diagnostic performance of significant predictors. P < 0.05 was considered significant.Results
A total of 27 participants (mean age ± standard deviation, 50.04±16.68) were enrolled in this study, 19 of whom were diagnosed with glioma and 8 with meningioma. Peritumoral T1 relaxation time and QSM value could distinguish between benign and malignant brain tumors (AUC = 0.804 and 0.795, respectively). The peritumoral T1 relaxation time and QSM value were significantly lower in glioma than in meningioma (P = 0.022 and 0.027, respectively). Among patients with glioma, peritumoral T2 star relaxation time could differentiate low-grade tumors from high-grade ones (AUC = 0.889), and peritumoral T2 star relaxation time in low-grade glioma was significantly longer than in high-grade glioma (P = 0.034).Discussion/Conclusion
In this study, the GRE-based sequence MULTIPLEX possessed great potential for multi-parametric MR imaging. MULTIPLEX MRI can effectively decrease the scan time while obtaining multi-parametric images and mappings. Besides, the simultaneous quantitative estimation of multiple MR parameters can reliably characterize and grade intracranial tumors. Only the parameters derived from the peritumoral zone exhibited significant differences between benign and malignant brain tumors, as well as between low-grade glioma and high-grade glioma. Previous studies suggested that the peritumoral region—the area immediately surrounding the tumor mass—may possess valuable outcome-related information. The peritumoral brain zone contains specific tumor and stromal cells that promote glioblastoma growth and invasion [8]. This recent progress in brain tumors and their peritumoral features will aid in the development of individualized targeted therapy and adjuvant glioma therapies after surgery. Further research with larger sample sizes is required to validate these preliminary results.Acknowledgements
No acknowledgement found.References
1. Gu, W., Fang, S., Hou, X., Ma, D., & Li, S. (2021). Exploring diagnostic performance of T2 mapping in diffuse glioma grading. Quantitative Imaging in Medicine and Surgery, 11(7), 2943.
2. Lasocki, A., Anjari, M., Ӧrs Kokurcan, S., & Thust, S. C. (2021). Conventional MRI features of adult diffuse glioma molecular subtypes: a systematic review. Neuroradiology, 63(3), 353-362.
3. West, H., Leach, J. L., Jones, B. V., Care, M., Radhakrishnan, R., Merrow, A. C., ... & Serai, S. D. (2017). Clinical validation of synthetic brain MRI in children: initial experience. Neuroradiology, 59(1), 43-50.
4. Chen, Y., Liu, S., Wang, Y., Kang, Y., & Haacke, E. M. (2018). STrategically Acquired Gradient Echo (STAGE) imaging, part I: Creating enhanced T1 contrast and standardized susceptibility weighted imaging and quantitative susceptibility mapping. Magnetic resonance imaging, 46, 130–139.
5. Ye, Y., Hu, J., Wu, D., & Haacke, E. M. (2013). Noncontrast-enhanced magnetic resonance angiography and venography imaging with enhanced angiography. Journal of magnetic resonance imaging: JMRI, 38(6), 1539–1548.
6. Blaimer, M., Breuer, F., Mueller, M., Heidemann, R. M., Griswold, M. A., & Jakob, P. M. (2004). SMASH, SENSE, PILS, GRAPPA: how to choose the optimal method. Topics in magnetic resonance imaging: TMRI, 15(4), 223–236.
7. Ye, Y., Lyu, J., Hu, Y., Zhang, Z., Xu, J., & Zhang, W. (2022). MULTI‐parametric MR imaging with fLEXible design (MULTIPLEX). Magnetic Resonance in Medicine, 87(2), 658-673.
8. Lemée, J. M., Clavreul, A., & Menei, P. (2015). Intratumoral heterogeneity in glioblastoma: don't forget the peritumoral brain zone. Neuro-oncology, 17(10), 1322-1332.
Figures