2350
qDWI-Morph: Motion-compensated quantitative Diffusion-Weighted MRI analysis for fetal lung maturity assessment1Technion, Haifa, Israel, 2Boston Children's Hospital, Boston, MA, United States
Synopsis
Keywords: Fetal, Diffusion/other diffusion imaging techniques, fetal imaging
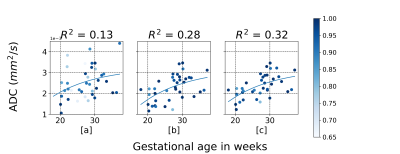
Quantitative analysis of fetal lung Diffusion Weighted MRI (DWI) data shows potential in providing quantitative imaging biomarkers for assessing fetal lung maturation. However, fetal motion during the acquisition impairs the accuracy and robustness of the analysis. We introduce qDWI-morph, an unsupervised deep-neural network architecture for motion correction and quantitative DWI (qDWI) analysis. We simultaneously estimate the qDWI parameters and the motion model by minimizing a bio-physically-informed loss. The qDWI-morph achieved an improved correlation between qDWI parameters in the fetal lung with the gestational age (R-squared=0.32) over baseline analysis without motion correction (R-squared=0.13) and our network with registration loss solely (R-squared=0.28).Introduction
The ability to accurately assess lung maturation before delivery is critical as newborns with inadequate in-utero lung development are at risk for post-natal respiratory failure or death [3]. The currently available non-invasive imaging modalities are limited to structural evaluation [4] rather than functional analysis of the fetal lung parenchyma, which is critical for maturity assessment. Changes in the overall tissue diffusivity are thought to serve as a functional indicator of lung development since, during gestation, there is a significant increase in pulmonary blood flow and perfusion.Diffusion-weighted MRI (DWI) is a non-invasive imaging technique sensitive to the random movement of individual water molecules in the tissue. Quantitative analysis of the DWI data (qDWI) has been suggested previously for functional assessment of fetal lung parenchyma development [5,1].
However, qDWI analysis is intrinsically highly susceptible to gross motion during the DWI data acquisition [1]. Hence, the irregular and unpredictable movement of the fetus, in addition to the maternal respiratory and abdominal motion, causes misalignment between the different image volumes acquired as part of the DWI acquisition. This motion impairs the accuracy and robustness of the ADC estimation [2]. We introduce qDWI-morph, an unsupervised deep-neural network architecture coupled with a bio-physically-informed loss for motion correction and quantitative DWI (qDWI) analysis.
Methods
Data: Legacy fetal DWI data was used in the study. The data acquisition was performed on a Siemens 3T Skyra scanner equipped with an 18-channel body matrix coil. Each patient was scanned with a multi-slice, single shot, echo-planar imaging (EPI) sequence that was used to acquire diffusion-weighted scans of the lungs. The in-plane resolution was 2.5mm × 2.5mm for each study, and the slice thickness was set at 3mm. Echo time (TE) was 60ms, whereas repetition time (TR) ranged from 2s to 4.4s depending on the number of slices required to cover the lungs. Each patient was scanned with 6 different b-values (0, 50, 100, 200, 400, 600 sec/mm2) in axial and coronal planes. A region of interest (ROI) was manually drawn for each case in the right lung [1]. We cropped each image to a shape: $$$96 \times 96 \times 16$$$ and normalized it by the maximal value at $$$S_0$$$. The data includes 38 cases with minor misalignments between the different b-values image volumes.Algorithms: We mathematically formalized a simultaneous qDWI analysis and motion compensation as an optimization problem. Since direct optimization is challenging, we introduced qDWI-morph, a self-supervised deep-neural network architecture, to solve it. Our approach couples a registration sub-network with a qDWI model-fitting sub-network (Fig.1). For the qDWI sub-network, we described the signal using a mono-exponential signal decay model with a decay rate that depends on the voxel's apparent diffusion coefficient (ADC). We simultaneously estimated the ADC and the motion model by minimizing a bio-physically-informed loss function integrating a registration loss and a model fitting quality loss. The input to our model is a batch of 3D different b-value images from the same patient, with their corresponding b-values. The output comprises a set of motion-corrected images forced pixel-wise to the mono-exponential model. Our method is iterative, where the motion-corrected images from the previous iteration enter as input to the next iteration until convergence. We solved the optimization problem for each case separately, so no training process was involved.
Experiments: We calculated average ADC values for the ROI using: 1) iterative-reweighted least-squares without any motion compensation, 2) qDWI-morph without model-fitting quality loss, and 3) qDWI-Morph with our proposed bio-physically-informed loss combining both a conventional registration loss and a model fitting quality loss. We used an exponential saturation model suggested by Afacan et al. [1] to analyze the correlation between the ADC and the GA. We assessed the following: 1) the effect of qDWI motion compensation on the correlation between the ADC parameter and GA, 2) the contribution of our proposed model fitting quality loss in addition to the registration loss in improving the observed correlation between the ADC parameter and GA.
Results
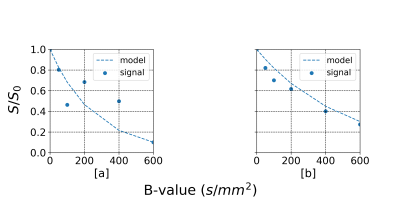
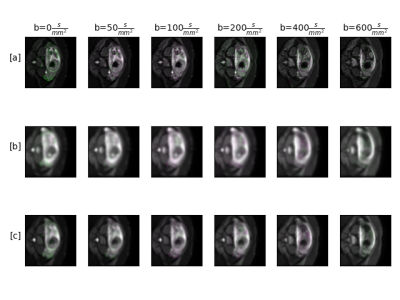
Fig.2 summarized the correlations between the averaged ADC in the fetus's lung and the GA. The baseline approach without motion compensation achieved an overall correlation of $$$R^2 = 0.13$$$. Our qDWI-Morph with a conventional registration loss achieved a correlation of $$$R2 = 0.28$$$. The addition of our bio-physically inspired loss further improve the correlation to $$$R^2 = 0.32$$$. Fig.3 presents the signal decay along with the fitted model with and without motion compensation. Fig.4 depicts the effect of the motion compensation on a representative case for the different methods.Conclusions
We introduced qDWI-Morph, an unsupervised DNN model for simultaneous motion compensation and qDWI analysis. We also proposed a bio-physically-informed loss function incorporating registration loss with qDWI model fitting loss for DNN optimization. Our experiments demonstrated the added-value of the proposed bio-physically-informed loss for fetal lung maturity assessment. The proposed approach can potentially improve our ability to quantify DWI signal decay model parameter in cases with motion.Acknowledgements
This research was supported in part by a grant from the United States-Israel Binational Science Foundation (BSF), Jerusalem, IsraelReferences
[1] Afacan, O., Gholipour, A., Mulkern, R.V., Barnewolt, C.E., Estroff, J.A., Connolly, S.A., Parad, R.B., Bairdain, S., Warfield, S.K.: Fetal lung apparent diffusion coefficient measurement using diffusion-weighted MRI at 3 Tesla: Correlation with gestational age. Journal of Magnetic Resonance Imaging 44(6), 1650–1655 (12 2016). https://doi.org/10.1002/jmri.25294
[2] Kurugol, S., Freiman, M., Afacan, O., Domachevsky, L., Perez-Rossello, J.M., Callahan, M.J., Warfield, S.K.: Motion-robust parameter estimation in abdominal diffusion-weighted MRI by simultaneous image registration and model estimation. Medical Image Analysis 39, 124–132 (7 2017). https://doi.org/10.1016/J.MEDIA.2017.04.006
[3] Lakshminrusimha, S., Keszler, M.: Persistent pulmonary hypertension of the newborn. Neoreviews 16(12), e680–e692 (2015)
[4] Moeglin, D., Talmant, C., Duyme, M., Lopez, A.C.: Fetal lung volumetry using two- and three-dimensional ultrasound. Ultrasound in Obstetrics & Gynecology 25(2), 119–127 (2 2005). https://doi.org/10.1002/UOG.1799, https://onlinelibrary.wiley.com/doi/full/10.1002/uog.1799 https://onlinelibrary.wiley.com/doi/abs/10.1002/uog.1799 https://obgyn.onlinelibrary.wiley.com/doi/10.1002/uog.1799
[5] Moore, R., Strachan, B., Tyler, D., Baker, P., Gowland, P.: In vivo diffusion measurements as an indication of fetal lung maturation using echo planar imaging at 0.5 t. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 45(2), 247–253 (2001)
Figures