2344
Ultra-high field characterisation of resting state networks in the neonatal brain1Department of Perinatal Imaging, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Guy's and St Thomas' NHS Foundation Trust, London, United Kingdom, 3MRC Centre for Neurodevelopmental Disorders, King's College London, London, United Kingdom, 4London Collaborative Ultra high field System (LoCUS), Kings College London, London, United Kingdom, 5MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
Keywords: Neonatal, fMRI (resting state)
Acquiring BOLD fMRI data at ultra-high field offers marked gains in sensitivity and spatial specificity including within cortical layers and distinct subcortical nuclei. We describe the first pilot data demonstrating feasibility of characterizing resting state networks in the neonatal brain using a 7 Tesla system. In 3 neonates imaged at full term, we show that in addition to the canonical networks seen at standard field strengths, ultra-high field fMRI enables network delineation with higher spatial specificity, including better localization to the cortex and with definition of individual networks corresponding to distinct anatomical regions and tissues.Introduction
Imaging at ultra-high field (UHF) offers marked gains in signal-to-noise ratio, particularly where contrast is dependent on magnetic susceptibility such as in Blood Oxygen Level Dependent (BOLD) functional MRI (fMRI) 1. This enables fine-scale studies of the brain’s functional architecture at higher resolution and sensitivity compared to standard field strengths, including delineation of activity within cortical columns, layers and specific deep grey matter nuclei 2. The ability to characterize such activity in the neonatal brain is compelling, as this is a crucial time for the establishment of the brain’s life-long framework of functional connectivity, with studies at 3T demonstrating rapid maturation of resting state networks in the time leading up to birth 3. Moreover, early-life alterations in functional connectivity due to environmental influences such as preterm birth persist into later life and correlate with adverse neurodevelopmental outcome 4. However, acquiring fMRI data at UHF from this fragile population poses several practical and acquisition challenges, and has therefore not been previously done. We aimed to explore the possible gains in sensitivity by delineating neonatal resting state networks using 7T fMRI.Methods
Data were acquired from 3 neonates (aged 37+6, 40+3, 41+0 weeks postmenstrual age (PMA)) following informed parental consent (NHS REC approval: 19/LO/1384) using a 1TX-32RX head coil (Nova Medical, Wilmington, MA, USA) with a modified safety model to enforce more conservative limits 5 and a 7T scanner (MAGNETOM Terra, Siemens Healthineers, Erlangen, Germany) at the LoCUS MRI Unit, St Thomas’ Hospital London. Infants were studied in natural sleep following feeding, with hearing protection (dental putty in the external auditory meatus and foam cushioning) and monitoring of their vital signs (oxygen saturation, heart rate, axillary temperature) throughout the scanning session. Studies were supervised by 2 members of clinical staff trained in neonatal resuscitation. There were no adverse events during these studies.Images were acquired with a single-band gradient echo EPI sequence with parameters: resolution: 1.95*1.95mm resolution; 40 2mm interleaved slices; TE/TR: 43ms/2930ms; bandwidth 1954Hz/pixel; total acquisition time 7m27s. Additional high resolution (0.6*0.6*1.2mm) T2-weighted images were also acquired for clinical reporting and image registration purposes. fMRI data analysis was performed using FSL 6, with standard preprocessing steps including rigid body motion correction, high pass filtering (cut-off 150s), spatial smoothing 3mm FWHM, and slice timing correction. Resting state networks were subsequently identified using Independent Component Analysis (ICA) as implemented in MELODIC with automated dimensionality estimation 7. Independent components were identified as resting state networks by reviewing their spatial representations and low frequency temporal characteristics.
Results
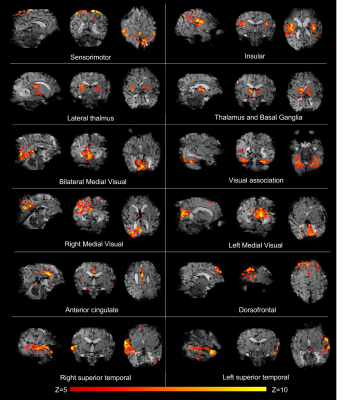
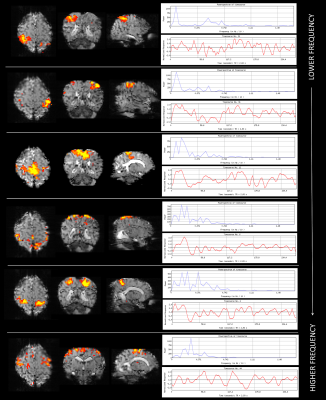
Data were successfully acquired in all 3 infants, with a complement of canonical resting state networks identified in each subject corresponding to known spatial distributions of coherent fluctuations in resting brain activity 3,7. These included the medial and lateral sensorimotor, anterior cingulate, dorso-frontal, medial and associative visual, insular, basal ganglia, thalami, and temporal networks (figure 1). In contrast to networks described in the literature at 3T which localize to brain regions but not to distinct tissue layers 3, specific neonatal resting state networks identified at 7T were seen to be spatially restricted to the superficial cortex, most notably in the medial visual and sensorimotor networks. In addition, the sensorimotor network contained several distinct “sub-networks”, with others localizing to deeper regions beyond the superficial cortex and to specific somatotopic regions (and their contralateral homologues) (figure 2).Discussion and Conclusions
We describe the feasibility of detailed characterization of emerging resting state networks in the neonatal brain at UHF and demonstrate the additional benefits that this enables in sensitivity. To our knowledge, this is the first such study in this population. In the described pilot work, we have used basic acquisition parameters and minimal pre-processing to establish feasibility. Later studies will likely benefit from the rapid advances in UHF fMRI acquisition which can markedly improve temporal and spatial (to the level of cortical layers 8) resolution through in-plane and multi-slice acceleration. Even without these tools, we demonstrate that acquiring fMRI data from neonates at 7T allows delineation of distinct “sub-networks” underlying the larger canonical networks, and these can be specifically localized to distinct tissue types and brain regions. Sub-network detection may allow finer grain analysis of developmental processes, which could provide new insights about the maturational role of transient structures such as the subplate in the maturation of the cortex and its associated network architecture 9. UHF fMRI could also help to resolve the uncertainty that remains about the relationship between the emergence of functional activity, the developing vasculature and the relative maturity of the underlying neurovascular coupling 10. It could also further improve understanding the effects of acquired neonatal brain injury, through unpicking of the pathophysiological mechanisms underlying resultant disruptions in long-term functional connectivity, which has both prognostic and therapeutic implications.fMRI at UHF offers marked gains in sensitivity and specificity, which when applied to the neonatal population holds great potential for providing new insights into the emergence of the brain’s functional architecture. We demonstrate both that neonatal fMRI is feasible at 7T and that it can provide detailed characterization of resting state networks in the developing brain.
Acknowledgements
This work was supported by a project grant awarded by Action Medical Research [GN2728], a Wellcome Trust Collaboration in science award [WT201526/Z/16/Z], by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health fand Social Care. TA was supported by funding from a Medical Research Council (MRC) Translation Support Award [MR/V036874/1]. ADE and TA received funding support from the MRC Centre for Neurodevelopmental Disorders, King’s College London [MR/N026063/1].References
[1]. Veissman O, Polimeni R. High-resolution fMRI at 7 Tesla: challenges, promises and recent developments for individual-focused fMRI studies. Current Opinion in Behavioral Sciences 2021; 40: 96-104.
[2]. Dumoulin SO, Fracasso A, van der Zwaag W, Siero JCW, Petridou N. Ultra-high field MRI: advancing systems neuroscience towards mesoscopic human brain function. Neuroimage 2018; 168: 345-57.
[3]. Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE et al. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci U S A 2010; 107(46): 20015-20.
[4]. Batalle D, Edwards AD, O'Muircheartaigh J Annual Research Review: Not just a small adult brain: understanding later neurodevelopment through imaging the neonatal brain. J Child Psychol Psychiatry 2018; 59: 350-371.
[5]. Malik SJ, Hand JW, Satnarine R, Price AN, Hajnal JV. Specific absorption rate and temperature in neonate models resulting from exposure to a 7T head coil. Magnetic Resonance in Medicine 2021 ; 86: 1299–313.
[6]. Jenkinson M, Beckmann CF, Behrens TE, et al. FSL. Neuroimage. 2012; 62: 782-90.
[7]. Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences 2005; 260(1457): 1001-1013.
[8]. Huber L, Handwerker DA, Jangraw DC, et al. High-Resolution CBV-fMRI allows mapping of laminar activity and connectivity of cortical input and output in human M1. Neuron 2017; 96(6): 1253-63.
[9]. Kostovic I. The enigmatic fetal subplate compartment forms an early tangential cortical nexus and provides the framework for construction of cortical connectivity. Prog Neurobiol. 2020; 194: 101883.
[10]. Kozberg M, Hillman E. Neurovascular coupling and energy metabolism in the developing brain. Prog Brain Res. 2016; 225: 213–242.
Figures