2343
Associations between Maternal Depression during Pregnancy and Neonatal Brain Resting-State Functional Connectivity1Radiology, University of Arkansas for Medical Sciences, Little Rock, AR, United States, 2Pediatrics, University of Arkansas for Medical Sciences, Little Rock, AR, United States, 3Arkansas Children’s Nutrition Center, Little Rock, AR, United States, 4Arkansas Children’s Research Institute, Little Rock, AR, United States, 5Biomedical Sciences and Imaging, Cedars Sinai Medical Center, Los Angeles, CA, United States, 6Biomedical Imaging Research Institute, Cedars Sinai Medical Center, Los Angeles, CA, United States, 7Bioengineering, University of California at Los Angeles, Los Angeles, CA, United States
Synopsis
Keywords: Neonatal, fMRI (resting state)
This study reports associations between maternal depression symptoms during pregnancy and neonatal brain functional connectivity. The mothers self-rated their depression symptoms during pregnancy using Beck Depression Inventory-II, and their newborns underwent a brain MRI examination including structural 3D T1-weighted images and resting-state fMRI for functional connectivity measurements. Most participants scored in the minimal range for depressive symptoms. Significant negative associations between maternal depression symptoms at ~36 weeks of pregnancy and newborn functional connectivity were observed in multiple brain regions/networks, indicating a negative influence of antenatal depression symptoms on neonatal brain functional development even in women with low symptoms of depression.INTRODUCTION
Depression is common during pregnancy [1, 2] and the current COVID-19 pandemic further increased the prevalence [3]. In this study, we aimed to investigate whether there is an association between maternal depression symptoms at different time points during pregnancy and neonatal brain functional development measured by resting-state fMRI.METHODS
Forty six pregnant women were recruited from an existing cohort for this study. Inclusion criteria for the pregnant women were: second parity, singleton pregnancy, ≤10 weeks of pregnancy, ≥21 years of age, conceived without assisted fertility treatments. Exclusion criteria were: pre-existing medical conditions such as diabetes mellitus, seizure disorder, and serious psychiatric disorders; drug abuse issues or alcohol use or smoking during pregnancy; sexually transmitted diseases; medical complications developed during pregnancy such as gestational diabetes and pre-eclampsia. Additional exclusion criteria for their newborns were: born preterm (<37 weeks of gestation), have medical conditions or medication known to influence growth and development, or unable to complete a neonatal brain MRI examination. All women assessed their depression symptoms by Beck Depression Inventory-II (BDI-II) at three time points during pregnancy (~12 weeks, ~24 weeks and ~36 weeks of gestation, respectively). The Beck Depression Inventory-II [4] is a 21 item self-report inventory measuring the severity of depression symptoms in adolescents and adults. Subject are asked to rate their feeling/behaviors during the past two week time period. Scores range from 0 to 63. Higher scores indicate higher ratings of depression symptoms. This measure is widely used in clinical and research and has good reliability and validity. At ~2 weeks of postnatal age, all newborns underwent an MRI examination of the brain on a Philips Acheiva 1.5T scanner during natural sleep without sedation, using a neonatal brain protocol which included a 3D T1-weighted sequence with a resolution of 1x1x1mm for structural MRI, and an EPI sequence with a resolution of 1.25x1.25x4mm3 and 150 dynamics for resting state fMRI (RS-fMRI). In the end, 43 mother-newborn dyads had both BDI-II scores and valid MRI images and were included for this study.The imaging preprocessing steps were similar to a previous publication [5]. Briefly, the resting-state functional MRI data were motion corrected, nuisance signal regressed and bandpass filtered. Specifically, the rigid-body motion was corrected using FSL [6] function mcflirt, and further scrubbing for those volumes was applied, with frame-wise displacement > 0.3mm or fewer than 5 volumes between the scrubbed volumes removed/scrubbed from the data. Three RS-fMRI dataset with less than 102 volumes after scrubbing were excluded. The nuisance regressors included the six motion parameters from mcflirt (white matter, cerebral spinal fluid signals), and their derivative, quadratic and squared derivative terms. The bandpass was implemented using AFNI (0.01-0.08Hz) [7]. The RS-fMRI data were aligned to UNC neonate template [8] using a combination of functional-to-anatomical and anatomical-to-template by ANTS [9]. The data were further smoothed using a Gussian kernel of 6mm full width at half maximum and truncated to 102 volumes withal. Finally, the global signal was extracted using grey matter regions defined by template and regressed from the data. Functional connectivity measurements were calculated by conducting the correlation matrix from the average time series extracted for each region in the template. The Pearson’s correlation coefficients (r) were then Fisher-z transformed, resulting in functional connectivity scores (z) for each subject.
Spearman’s rank partial correlation tests were used to evaluate potential associations between BDI-II scores at different time points during pregnancy and neonatal functional connectivity between 90 different brain regions, with sex and gestational age controlled. Bonferroni correction was applied for the multiple correction for the 90 brain regions, and correlations with corrected P values ≤ 0.05 were regarded as significant. All statistics analyses were implemented in Python (version 3.6) and Matlab software (Version R2018b).
RESULTS
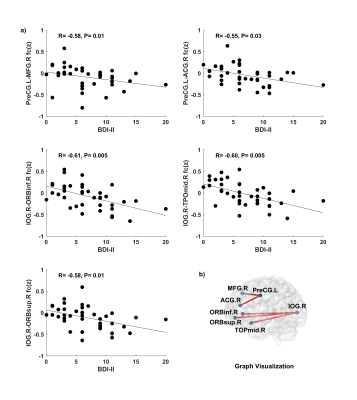
Significant negative correlations (corrected P ≤ 0.05) between maternal BDI-II scores at ~36 weeks of pregnancy and neonatal resting-state functional connectivity were identified in 5 region pairs: left precentral gyrus and right middle frontal gyrus (R = -0.58, P = 0.01), left precentral gyrus and right anterior cingulum gyrus (R = -0.55, P = 0.03), right inferior orbital frontal gyrus and right inferior occipital gyrus (R = -0.61, P = 0.005), right inferior occipital gyrus and right middle temporal pole gyrus (R = -0.60, P = 0.005), and right superior orbital frontal gyrus and right inferior occipital gyrus (R = -0.58, P = 0.01) (Figure 1).CONCLUSIONS
Our results show that more maternal depression symptoms during late pregnancy are associated with lower neonatal brain resting-state functional connectivity in multiple brain regions/networks, indicating a negative impact on offspring brain development.Acknowledgements
This project was supported in part by NIH 1R01HD099099 and USDA-ARS 6026-51000-012-06S.References
1. Gaynes, B.N., et al., Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ), 2005(119): p. 1-8.
2. Van Niel, M.S. and J.L. Payne, Perinatal depression: A review. Cleve Clin J Med, 2020. 87(5): p. 273-277.
3. Liu, C.H., C. Erdei, and L. Mittal, Risk factors for depression, anxiety, and PTSD symptoms in perinatal women during the COVID-19 Pandemic. Psychiatry Res, 2021. 295: p. 113552.
4. Beck, A.T., R.A. Steer, and G. Brown, 2011.
5. Chen, H.T., et al., Developmental heatmaps of brain functional connectivity from newborns to 6-year-olds. Developmental Cognitive Neuroscience, 2021. 50.
6. Jenkinson, M., et al., Fsl. Neuroimage, 2012. 62(2): p. 782-90.
7. Cox, R.W., AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res, 1996. 29(3): p. 162-73.
8. Shi, F., et al., Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One, 2011. 6(4): p. e18746.
9. Avants, B.B., et al., A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 2011. 54(3): p. 2033-44.
Figures