2337
A quantitative study on the growth patterns of vertebrae in utero fetuses in second-third trimester:a prospective study based “black-bone” MRI1Shandong Provincial Hospital Affiliated to Shandong First Medical University, jinan, China, 2MR Collaboration, Siemens Healthineers Ltd, Beijing, China. Email: jinxia.zhu@siemens-healthineers.com, beijing, China
Synopsis
Keywords: Fetal, Normal development
Imaging the anatomy of the spine and its relevant pathology is clinically important for the early identification of spinal malformations and anomalous osseous development. our study applied MRI-based “black-bone” sequence to elucidate the growth dynamics of vertebrae ossification center in utero fetuses in second-third trimester. We found that the fetal vertebrae were correlated with gestational age and follow a certain formula, providing the existing literature with completely novel quantitative data.Introduction
The development of vertebral ossification center is an important index to judge gestational age and to evaluate fetal maturity[1]. Abnormal or delayed development of the ossification center may lead to congenital vertebral dysplasia[2-4]. Hence, detailed knowledge on the normative growth of the fetal vertebrae is of great relevance in the prenatal diagnosis of its abnormalities and vertebral anomalies.At present, studies on the development of fetal vertebral ossification center mostly focus on fetal specimens [5] [6-8] [9, 10], However, specimens soaked by formalin may cause protein denaturation and tissue dehydration, and the bone structure may be decalcified when stored for a long time[11].
Ultrasonography (US) has been routinely used to assess the development of fetal spine in utero [5, 12-15], but certain fetal and/or maternal factors [16] may adversely affect the quality of US images. In recent years, with the improvement of prenatal MRI, especially the MRI "black bone" sequence, namely the optimized susceptibility-weighted imaging (SWI), yields high contrast between fetal vertebra and soft tissues, but lower contrast between different soft tissues, and provides a good definition and high image quality of fetal vertebrae ossification center [17]. But for now, little has been known in the literature on morphometric values for fetal vertebral ossification center in vivo and in utero.
In this work, we applied MRI-based “black-bone” SWI sequence to measure the morphological parameters of the fetal vertebral centrum ossification centers (COC) in the middle-late gestational ages, and to plot the growth and development trajectory of the vertebrae, providing MRI age-specific reference values for fetal vertebrae in vivo and in utero.
Methods
Fetus in the second-third trimester with normal vertebrae development on ultrasound who underwent 1.5-T magnetic resonance imaging (MRI) (Siemens, Amira Shenzhen Magnetic Resonance, Ltd., Shenzhen, China) were included. MRI-"black bone" SWI sequence was performed at the 3 orientation of the fetal spine. The following morphometric parameters of the C4、T6、L3、S1 vertebrae COC were measured: transverse diameter, sagittal diameter, height, cross-sectional area and volume. Pearson correlations were used to correlate the C4, T6, L3, S1 COC measurements with GA. Linear and nonlinear regression analysis was used to derive the best-fit curve for each parameters versus gestational age.Results
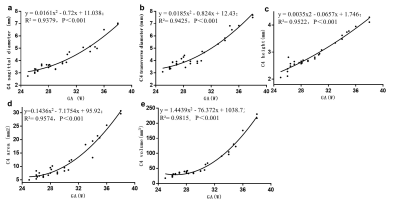
A total of 127 participants were recruited, mean age 28.7±5.8y, mean GA 29.4±3.9w. Of these, C4 COC was 30; T6 COC was 58; L3 COC was 92; S1 COC was 62. Fetal spine in utero with global curvature was kyphosis, presenting two primary curves (thoracic and sacral kyphosis) and two secondary curves (cervical and lumbar lordosis); the vertebral body shape is regular in round, oval, or square (figure 1). The sagittal diameter, transverse diameter, height, area and volume increase of C4, T6, L3 and S1 ossification centers were significantly correlated with gestational age (P<0.001) (figure 2-5).Dicussion
Identifying maturity of fetal vertebral development or congenital anomalies e.g. hemivertebrae, butterfly vertebrae in utero fetuses were mostly based on reduced dimensions of bones in relation to gestational age and abnormal morphological features or bone mineralization. Our study showed MRI-based “black-bone” SWI sequence was able to characterize the morphological parameter of C4, T6, L3, S1 COC in vivo, providing the existing literature with completely novel quantitative data. Growth dynamics of the vertebrae COC in sagittal diameter, transverse diameter and height development in our study showed exponential (C4, T6, L3) and linear (S1), and both the area and volume was quadratic polynomial, and the coefficients of the quadratic terms are positive. This indicates that the fetal vertebral COC (21-39w) in our study grew slowly in the early stage, while grew faster with the increase of gestational age. The development trajectory evaluation of the fetal vertebral COC in our study involved one-dimensional (sagittal diameter, transverse diameter, height), two-dimensional (area), and three-dimensional (volume) perspectives. For the first time, prenatal MRI was used to comprehensively evaluate the growth and development dynamics of intrauterine fetal vertebral bodies from a new perspective.Conclusion
This study showed that the growth and development of C4, T6, L3 and S1 COC in the middle-late trimester had a good correlation with gestational age, providing reference values of MRI and may be useful in the prenatal diagnosis of fetal vertebral anomalies.Acknowledgements
No acknowledgement found.References
[1] SAN ROMáN P, PALMA J C, OTEO M D, et al. Skeletal maturation determined by cervical vertebrae development [J]. European journal of orthodontics, 2002, 24(3): 303-11.
[2] NAFFAA L, IRANI N, SAADE C, et al. Congenital anomalies of lumbosacral spine: A pictorial review [J]. Journal of Medical Imaging and Radiation Oncology, 2017, 61(2): 216-24.
[3] GRIMME J D, CASTILLO M. Congenital anomalies of the spine [J]. Neuroimaging Clin N Am, 2007, 17(1): 1-16.
[4] LIU S B, DE BERITTO T V. Congenital Cervical Spondyloptosis in the Neonate: A Prenatal Diagnosis [J]. Pediatr Ann, 2020, 49(7): e313-e8.
[5] BUDORICK N E, PRETORIUS D H, GRAFE M R, et al. Ossification of the fetal spine [J]. Radiology, 1991, 181(2): 561-5.
[6] SZPINDA M, BAUMGART M, SZPINDA A, et al. Cross-sectional study of the ossification center of the C1-S5 vertebral bodies [J]. Surg Radiol Anat, 2013, 35(5): 395-402.
[7] BAUMGART M, WISNIEWSKI M, GRZONKOWSKA M, et al. Quantitative anatomy of the ilium's primary ossification center in the human fetus [J]. Surg Radiol Anat, 2018, 40(9): 1047-54.
[8] SZPINDA M, BAUMGART M, SZPINDA A, et al. New patterns of the growing L3 vertebra and its 3 ossification centers in human fetuses - a CT, digital, and statistical study [J]. Medical science monitor basic research, 2013, 19: 169-80.
[9] WIDJAJA E, WHITBY E H, COHEN M, et al. Post-mortem MRI of the foetal spine and spinal cord [J]. Clin Radiol, 2006, 61(8): 679-85.
[10] JIAN N, TIAN M M, XIAO L X, et al. Normal development of sacrococcygeal centrum ossification centers in the fetal spine: a postmortem magnetic resonance imaging study [J]. Neuroradiology, 2018, 60(8): 821-33.
[11] BIRKL C, LANGKAMMER C, GOLOB-SCHWARZL N, et al. Effects of formalin fixation and temperature on MR relaxation times in the human brain [J]. NMR Biomed, 2016, 29(4): 458-65.
[12] SCHILD R L, WALLNY T, FIMMERS R, et al. Fetal lumbar spine volumetry by three-dimensional ultrasound [J]. Ultrasound Obstet Gynecol, 1999, 13(5): 335-9.
[13] WAX J R, WATSON W J, MILLER R C, et al. Prenatal sonographic diagnosis of hemivertebrae: associations and outcomes [J]. J Ultrasound Med, 2008, 27(7): 1023-7.
[14] SCHILD R L, WALLNY T, FIMMERS R, et al. The size of the fetal thoracolumbar spine: a three-dimensional ultrasound study [J]. Ultrasound Obstet Gynecol, 2000, 16(5): 468-72.
[15] WEISZ B, ACHIRON R, SCHINDLER A, et al. Prenatal sonographic diagnosis of hemivertebra [J]. J Ultrasound Med, 2004, 23(6): 853-7.
[16] HENDLER I, BLACKWELL S C, BUJOLD E, et al. Suboptimal second-trimester ultrasonographic visualization of the fetal heart in obese women: should we repeat the examination? [J]. J Ultrasound Med, 2005, 24(9): 1205-9; quiz 10-1.
[17] CAI X, CHEN X, WANG J, et al. Susceptibility-weighted imaging to evaluate normal and abnormal vertebrae in fetuses: A preliminary study [J]. Prenat Diagn, 2022, 42(11): 1398-408.
Figures