2331
Prediction of IDH status of Glioma using Diffusion Tensor Imaging and Clinical features1The first affiliated hospital of Xi'an Jiaotong University, Xi'An, China, 2Hospital of Stomatology Xi'an Jiaotong University, Xi'an, China, 3The first affiliated hospital of Xi'an Jiaotong University, Xi'an, China, 4The first affiliated hospital of Xi'an Jiaotong University, XI'an, China
Synopsis
Keywords: Tumors, Diffusion Tensor Imaging, glioma
Isocitrate dehydrogenase (IDH) is critical to prognosis of glioma. While, reliable techniques for preoperative assessment of IDH status remain scarce. In this study, we investigated mean diffusivity (MD) and anisotropy fraction (FA) using Diffusion Tensor Imaging (DTI) combined with the clinical features to predict IDH status. Our results found significant differences in FAmean/FAnawm, MDmin, NLR, and age between IDH mutant and IDH wild groups. The model incorporating FAmean/FAnawm, MDmin, NLR, and age predicted IDH status with area under ROC curve of 0.85, 95% CI: 74.3%~95.7%. Our findings suggested that DTI combined with clinical features can non-invasively prediction of IDH status.Introduction
Glioma is the most common intracranial malignant tumor. As an independent prognostic factor of glioma, isocitrate dehydrogenase (IDH) plays an important role in the diagnosis, treatment and prognosis of glioma [1,2]. Diffusion tensor imaging (DTI) is a routinely used in brain tumor, it functionally reflects the diffusion ability of water molecules in tumor tissues. Previous studies only focused on imaging manifestations predicting tumor status, there are few studies combine imaging manifestations with clinical features to predict IDH status. In this study, the quantitative parameters of DTI combined with serum neutrophils/lymphocytes (NLR) and age were used to noninvasively predict the status of IDH before surgery.Methods
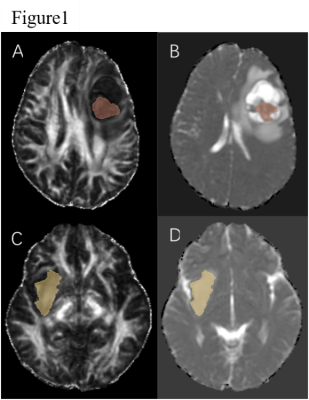
47 glioma patients confirmed by pathology and IDH gene test were eligible for the study, including 20 cases of IDH mutant type and 27 cases of IDH wild type. Among all patients, there were 29 males and 18 females, with an average age of 48.00±12.17 years old. FSL software was used to process DTI images. 3DSlicer software was used to delineate ROIs in solid part of the tumor and contralateral normal appeared white matter (NAWM). After registered FLAIR images with MD and FA images, quantitative analysis of DTI parameters in ROIs was performed to obtain the minimum MD (MDmin), the mean MD/the normal appeared white matter MD (MDmean/MDnawm), the minimum FA(FAmin), the mean FA/the normal appeared white matter FA(FAmean/FAnawm), respectively. The maximum diameter of the tumor was measured on FLAIR image, and the tumor was classified according to whether there were cysts. The preoperative serum neutrophils/lymphocytes (NLR) of the patients were calculated. The Student t test and Chi-square test were used to compare the differences of FA, MD, maximum tumor diameter, cysts, NLR and age in IDH mutant type and wild type glioma groups. The factors with statistical significance (p<0.05) were incorporated in the Logistic regression model. The predictive efficiency of the model was tested, the receiver operating curve (ROC) curve was drawn and the area under the curve (AUC) was calculated.Results
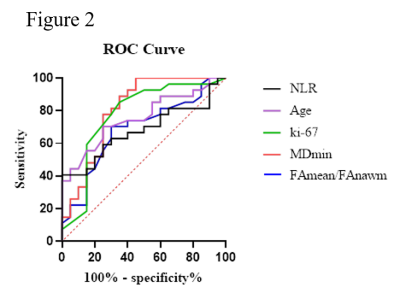
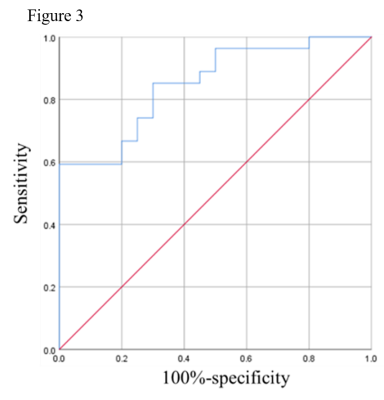
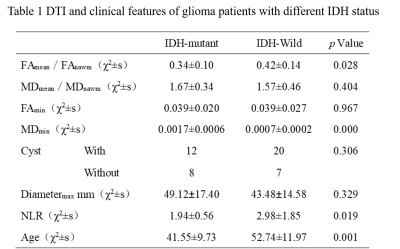
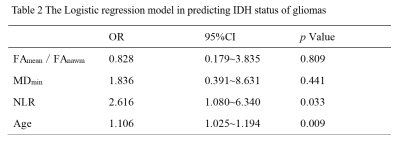
As Table 1 showed, there were significant differences in FAmean/FAnawm, MDmin, NLR and age between the IDH mutant type and IDH wild type glioma groups (p<0.05), among these parameters, MDmin had the best performance in predicting IDH (p=0.000, AUC=80.74%). However, there were no significant differences in MDmean/MDnawm, FAmin and cysts between the two groups (p>0.05). Then, FAmean/FAnawm, MDmin, NLR and age were incorporated in the Logistic regression model. The model showed good performance in predicting IDH status (AUC: 85.0%; 95% CI: 74.3%~95.7%).Discussion
Gliomas are the most common primary malignant brain tumors in adults. Recent studies have highlighted IDH status as an important prognostic factor for gliomas [1,2]. In this study, we compared FA and MD values of the solid part of gliomas in different IDH status. The results showed that MD values were significantly lower in IDH wild type gliomas compared with IDH mutant type gliomas. Lower MD of solid part of glioma may reflect the faster proliferation rate of IDH wild type glioma, it caused the higher density of cells, so the diffusion degree of water molecules was significantly limited [3,4]. Furthermore, our results indicated that the FA values of IDH wild type gliomas were significantly higher than that of IDH mutant type gliomas. However, previous studies found that the changes of FA in IDH wild and IDH mutant gliomas are controversial [5,6]. The differences between these findings suggest that the factors affecting FA values may involve more complex mechanism besides membrane integrity and tumor cell density changes. NLR is a widely used to evaluate preoperative inflammatory and survival. In addition, high NLR was associated with poorer overall survival (OS) and higher tumor grade [7]. In this study, NLR was significantly lower in IDH mutant type gliomas. The lower NLR may be caused by immunosuppression of IDH mutations, which also explains the longer survival of patients with IDH mutant type glioma. Age is considered to be a predictor of glioma IDH status, as studies have shown that patients with IDH wild glioma are usually older than those with IDH mutant glioma [8].Conclusion
In this study, the model combined DTI and clinical features showed a great predictive performance on IDH status of gliomas. It is expected to provide a non-invasive diagnosis of glioma and to guide clinical individualized treatment.Acknowledgements
No acknowledgement found.References
1. Gritsch S, Batchelor TT. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer. 2022 Jan 1;128(1):47-58.
2. Yang K, Wu Z. Glioma targeted therapy: insight into future of molecular approaches. Mol Cancer. 2022 Feb 8;21(1):39.
3. Maynard J, Okuchi S. World Health Organization Grade II/III Glioma Molecular Status: Prediction by MRI Morphologic Features and Apparent Diffusion Coefficient. Radiology. 2021 Jan;298(1): E61.
4. Xu Z, Ke C. Diagnostic performance between MR amide proton transfer (APT) and diffusion kurtosis imaging (DKI) in glioma grading and IDH mutation status prediction at 3 T. Eur J Radiol. 2021 Jan; 134:109466.
5. Jütten K, Weninger L. Dissociation of structural and functional connectomic coherence in glioma patients. Sci Rep. 2021 Aug 18;11(1):16790.
6. Huang Z, Lu C. Prediction of Lower Grade Insular Glioma Molecular Pathology Using Diffusion Tensor Imaging Metric-Based Histogram Parameters. Front Oncol. 2021 Mar 10; 11:627202.
7. Gomes Dos Santos A. Role of neutrophil-lymphocyte ratio as a predictive factor of glioma tumor grade: A systematic review. Crit Rev Oncol Hematol. 2021 Jul; 163:103372.
8.Zhou H, Vallières M, Bai HX, et al. MRI features predict survival and molecular markers in diffuse lower-grade gliomas. Neuro Oncol 2017;19(6):862–870.
Figures