2324
Analysis of fasting-induced metabolic changes in glioma tissue detected by non-invasive 1H/31P MR-Spectroscopy1Institute of Neuroradiology, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany, 2University Cancer Center Frankfurt (UCT), Frankfurt am Main, Germany, 3Frankfurt Cancer Institute (FCI), Frankfurt am Main, Germany, 4German Cancer Research Center (DKFZ) Heidelberg, Germany and German Cancer Consortium (DKTK), Partner Site Frankfurt/Mainz, Frankfurt am Main, Germany, 5Dr. Senckenberg Institute of Neurooncology, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany, 6Department of Neurosurgery, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany
Synopsis
Keywords: Tumors, Spectroscopy
Nutritional interventions such as fasting are currently under evaluation as anti-cancer treatment. In glioma patient cohorts, the feasibility and safety of fasting in addition to antitumor treatment has been shown. However, it is still unclear whether fasting exerts effects on the glioma tumor tissue at all, and whether fasting causes metabolic or immunological changes in the glioma microenvironment that could be exploited therapeutically. This is a report on the metabolic flexibility of tumor cells induced by a fasting cycle of 72 hours prior to biopsy or resection analyzed by MR-Spectroscopy (MRS) as part of the ERGO3-trial.
Introduction
Key to the integration of fasting into cancer treatment is the observation that healthy cells and tumor cells differ in their ability to adapt to a diet-induced nutrient-deprivation. While normal cells enter a self-maintenance mode and switch to alternative catabolic pathways, such as the oxidation of fatty acids or ketone bodies (KB), tumor cells harbor oncogenic mutations that seem to limit their metabolic flexibility.1 While normal cells of the CNS begin to utilize KBs as an energy source by the CNS when they reach a blood concentration of about 4 mmol/l,7 it is still unknown whether fasting exerts effects on the glioma tumor tissue at all. Neither is it known whether fasting causes metabolic or immunological changes in the glioma microenvironment that could be exploited therapeutically.2-6 A better understanding of diet-induced changes in the glioma tissue is crucial to successfully incorporate dietary interventions into current treatment options for glioma patients. The central contribution of this study is to assess these alterations by detailed in vivo and ex vivo analyses of the tumor tissue, and to help identify patient subgroups eligible for fasting as a therapeutic strategy. This is a report on the metabolic flexibility of tumor cells analyzed by MR-Spectroscopy (MRS) as part of the ERGO3-trial.Methods
The ERGO3 trial (Characterization of Metabolic Changes in the Glioma Tumor Tissue Induced by Transient Fasting; ClinicalTrials.gov Identifier: NCT04461938) was designed to analyze diet-induced changes in glioma tissue. Patients ≥ 18 years with MRI-suspected glioma and recommendation for biopsy/resection were eligible. The study intervention consisted of one fasting cycle of 72 hours prior to biopsy/resection (Figure 1). All study participants followed the same dietary intervention. Changes in metabolic parameters were assessed in serum, capillary blood and urine before, during and after the intervention. The analysis of the tumor tissue (post-surgery) included a standard neuropathological workup and additional metabolic analyses to assess the effects of fasting on tumor cells (metabolomics and RNA sequencing). MRS was performed prior to fasting and after 72 hours of fasting in order to non-invasively assess metabolic adaption of tumor cells.Examinations were performed on a clinical whole-body 3T MR Scanner (MAGNETOM Prisma, Siemens Healthineers, Erlangen) using a double-tuned 1H/31P volume head coil (RAPID Biomedical GmbH). The protocol included the following MRS sequences:
· 3D 1H decoupled 31P MRSI
· 2D 1H chemical shift imaging (CSI) semiLASER (TE 144)
Main target parameters of MRS were the detection of intracerebral ketone bodies (KB; acetone (Ac), acetoacetate (AcAc), β-hydroxybutyrate (βOHB)), changes in lactate, ATP/ADP concentrations and in intracellular pH (pHi).
Results
Twenty patients were examined with the MRS protocol at baseline and on day 3. 15 patients were diagnosed with IDH-wildtype glioblastoma, two with IDH-mutant astrocytoma, and two with IDH-mutant, 1p/19q-codeleted oligodendroglioma. One patient was diagnosed with cerebral metastasis and therefore excluded from the analysis. Four patients were excluded from standard data analysis due to an extensive line broadening caused by magnetic susceptibility gradients in the temporal lobe. In one patient 31P MRSI measurement failed at baseline. Metabolites were obtained in voxels selected from solid tumor and the contralateral hemisphere. Spectra were analyzed with LCModel, adding signals of 3-hydroxybutyrate (βOHB), acetone (Acn) and acetoacetate (AcAc) to a standard basis set simulated for the respective TE.From all patients included in the MRS analysis, 4/14 reached desired levels of blood ketosis (βOHB ³4 mmol/l). In 2 of those, βOHB was detected within tumor tissue by MRS on day 3 (Figure 2). MRSI results in patients with successful intervention did not show a significant depletion of ATP in tumor tissue from baseline to day 3. The tumor-specific alkaline pHi was maintained (Figure 3).
Discussion
Our findings suggest that fasting induces distinct metabolic alterations in tumor tissue while ATP levels as a measure of energy homeostasis appear unchanged. Tumor cells seem to maintain energy homeostasis even with reduced glucose levels. Significant blood ketosis was defined as βOHB levels ³ 4 mmol/l, a concentration close to the Michaelis constant for the monocarboxylate transporter, as AcAc and β-OHB as hydrophilic anions can merely cross the blood-brain barrier (BBB) that way. Only half of the patients with significant levels of blood ketosis had MRSI-detectable βOHB signals in the tumor tissue. Accordingly, reports on MRSI-detectability of KB are not consistent throughout literature and concentrations sometimes fail to show a correlation with serum or urine levels. This may be related to the rather small signal amplitudes of the KB, especially after a short dietary intervention (72 hours of fasting).8-12 Further reports on the ERGO3 study collective will include correlations with metabolic analyses of tumor tissue (post-surgery).Acknowledgements
KJW and SA were funded by the Mildred Scheel Career Center Frankfurt (Deutsche Krebshilfe).References
1. Nencioni, A., Caffa, I., Cortellino, S. & Longo, V. D. Fasting and cancer: molecular mechanisms and clinical application. Nat Rev Cancer 18, 707–719 (2018).
2. Lu, Z. et al. Fasting selectively blocks development of acute lymphoblastic leukemia via leptin-receptor upregulation. Nat Med 23, 79–90 (2017).
3. Lo Re, O. et al. Fasting inhibits hepatic stellate cells activation and potentiates anti-cancer activity of Sorafenib in hepatocellular cancer cells. J Cell Physiol 233, 1202–1212 (2018).
4. Lussier, D. M. et al. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer 16, 310 (2016).
5. Di Biase, S. et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 30, 136–146 (2016).
6. Pietrocola, F. et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell 30, 147–160 (2016).
7. Paoli, A., Rubini, A., Volek, J. S. & Grimaldi, K. A. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr 67, 789–796 (2013).
8. Seymour, K. J., Bluml, S., Sutherling, J., Sutherling, W. & Ross, B. D. Identification of cerebral acetone by 1H-MRS in patients with epilepsy controlled by ketogenic diet. MAGMA 8, 33–42 (1999).
9. Pan, J. W., Rothman, T. L., Behar, K. L., Stein, D. T. & Hetherington, H. P. Human brain beta-hydroxybutyrate and lactate increase in fasting-induced ketosis. J. Cereb. Blood Flow Metab. 20, 1502–1507 (2000).
10. Cecil, K. M., Mulkey, S. B., Ou, X. & Glasier, C. M. Brain ketones detected by proton magnetic resonance spectroscopy in an infant with Ohtahara syndrome treated with ketogenic diet. Pediatr Radiol 45, 133–137 (2015).
11. Berrington, A. et al. Cerebral Ketones Detected by 3T MR Spectroscopy in Patients with High-Grade Glioma on an Atkins-Based Diet. AJNR Am J Neuroradiol 40, 1908–1915 (2019).
12. Wenger, K. J. et al. Maintenance of Energy Homeostasis during Calorically Restricted Ketogenic Diet and Fasting-MR-Spectroscopic Insights from the ERGO2 Trial. Cancers (Basel) 12, (2020).
Figures
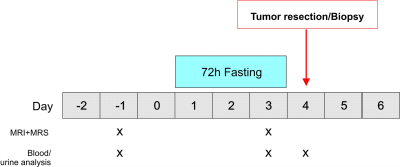
Figure 1. Study protocol. Patients with MRI-suspected glioma and recommendation for biopsy/resection are eligible for this study. The study intervention consists of one fasting cycle of 72 hours prior to biopsy/resection. All study participants follow the same dietary intervention. MRS is performed prior to fasting and after 72 hours of fasting in order to non-invasively assess metabolic flexibility of tumor cells.
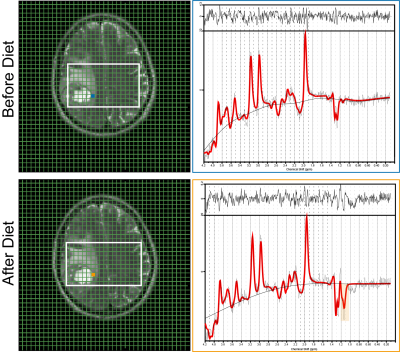
Figure 2. Left: Graphical user interface on T2-weighted MR images for two examinations (before and after dietary intervention) with a small box indicating the voxel used for spectroscopy analysis. Right: Corresponding spectra for the marked voxels. The original signals are presented in black, the LCModel fits in red. The 1.19-ppm βOHB signal arises from three protons but is a doublet due to J-coupling. The peak is highlighted in the orange box. There is no optimum TE to separate βOHB and Lac. However, they were resolvable at 3T using a TE of 144 ms.
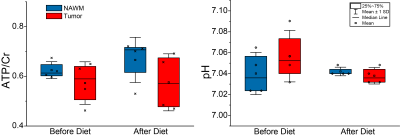
Figure 3. Box plots of ATP/Cr and pHi from tumor and NAWM at baseline and after dietary intervention (72 hours of fasting) that were acquired with 3T long (144 ms) TE MRSI.