2322
Comprehensive Analysis Of APTw Imaging In Diagnosis And Grading Of Gliomas – Bridging The Gap Between Imaging And Histopathological Outcomes1Radiodiagnosis, Apollo Hospitals Chennai, Chennai, India, 2Radiology, Apollo Hospitals, Chennai, India, 3Philips India Ltd, Gurgaon, India
Synopsis
Keywords: Tumors, CEST & MT, amide proton transfer
The utility of Amide Proton Transfer weighted imaging in preoperative grading of gliomas was studied. 64 patients with various primary intraaxial tumours were included in the study. Mean APTw was able to differentiate between IDH mutant grade 2 and grade 3 gliomas (astrocytomas and oligodendrogliomas) with statistical significance (p<0.05). APTw imaging can well delineate between the tumour core and the tumour periphery and the difference in the APT means between the two regions is statistically significant. APTw imaging also identifies grade 3 tumours misclassified as grade 2 on other sequences.INTRODUCTION
Remarkable advancements in glioma imaging have been made in recent years with the help of advanced imaging techniques like Susceptibility and Diffusion weighted imaging (SWI, DWI), spectroscopy, DSC (Dynamic Susceptibility Contrast) perfusion and ASL (arterial spin labelled) perfusion techniques. However, the grading and differentiation of gliomas from other malignant tumours of the brain such as lymphoma still poses a diagnostic dilemma in daily clinical practice. Eg: Extensive intratumoural hemorrhages can mask the lesion and impede the DSC perfusion analysis. Magnetic susceptibility artefacts can deter the analysis in EPI sequences. Amide Proton Transfer-weighted imaging (APTw)– a relatively novel molecular based technique is fundamentally different from other advanced MRI techniques. It reflects the mobile protein content within the tumour and is an indirect marker of cellular density.The objectives of the study are to determine the utility of APTw imaging:
1) To differentiate between IDH mutant grade 2 and grade 3 astrocytomas and oligodendrogliomas
2) To define the tumour extent
3) To differentiate high grade gliomas from primary CNS lymphoma.
METHODS
The study was commenced after the Institutional Ethics Committee approval with written informed consent obtained from all participants. 64 patients with primary intra axial brain tumour on imaging were included in the study. Studies with suboptimal techniques, non-glial intra-axial tumours, post-treatment follow up cases were excluded.The patients were imaged on a 3T scanner with a 16 channel receive-only head coil array. Both conventional - T1, T2, FLAIR and post contrast T1 sequences as well as advanced sequences such as DWI, SWI, ASL, DSC perfusion were acquired. A clinically approved APTw sequence was used and images were acquired in transverse oblique orientation parallel to the intercommissural line avoiding paranasal sinues (to avoid artifacts).
After histopathological confirmation, a retrospective analyses of the cases by 2 experienced radiologists (blinded to the histopathological diagnosis) was undertaken. Two sets of analyses were made. The analysis based on conventional and other advanced imaging techniques - DWI, SWI, ASL rCBF, DSC perfusion was termed ‘group 1’ and the analysis after including APTw was termed ‘group 2’. The two groups were compared.
RESULTS
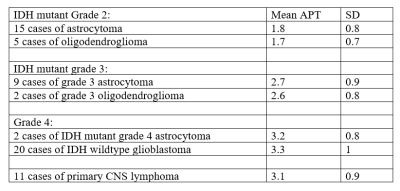
The mean APT values (expressed as %) along with standard deviation of the various tumours studied are given in table 1.The difference in mean APT between the IDH mutant grade 2 and grade 3 astrocytomas and oligodendrogliomas was statistically significant (p<0.05).
The difference between IDH mutant grade 3 astrocytomas and grade 4 tumours (IDH wildtype glioblastoma and IDH mutant grade 4 astrocytoma) was also significant (p<0.05).
9 out of 21 (42%) of the IDH mutant grade 3 astrocytoma were misclassified as grade 2 tumour in group A. In group B, using a mean APT cut-off value as 2.5% (suggested by previous researches(1) these tumours were correctly classified as grade 3.
No significant difference was seen in the mean APT value of IDH mutant grade 2 oligodendroglioma versus astrocytoma and IDH mutant grade 3 oligodendroglioma versus grade 3 astrocytomas.
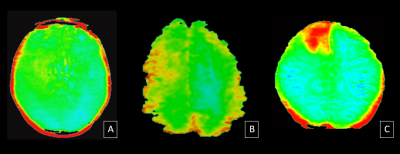
In 38 out of 53 cases (71%), higher mean APT values were seen in the central region of the tumour and lower mean APT values in the periphery of the tumour. This was designated as tumour core and tumour periphery respectively and the difference in their mean APT value was significant (p=0.04).
There was no statistically significant difference in the mean APT value between grade 4 gliomas and CNS lymphoma (p>0.05, student’s t-test).
DISCUSSION
Using APTw, a reliable differentiation between IDH mutant grade 2 and grade 3 astrocytomas can be made which is often a difficult call to make on imaging. This is in concordance with previous studies (1,2).APTw can correctly classify 42% of IDH mutant grade 3 astrocytomas which were initially misclassified as grade 2 due to their relatively indolent appearance on other sequences. This suggests that addition of APT leads to a better correlation with the histopathological grading of tumours.
The distinction between grade 2 and grade 3 is particularly important as it leads to difference in treatment planning and management (3).
APTw imaging can well delineate the tumour core from tumour periphery. This has important implications for surgical and radiotherapy planning.
Mean APT values cannot be used to differentiate high grade gliomas from CNS lymphomas. This is in discordance to few previous studies that suggest otherwise. (4)
CONCLUSION
- Mean APT values can differentiate IDH mutant grade 2 from grade 3 astrocytomas and oligodendrogliomas.
- 42% of grade 3 astrocytomas were initially misclassifed as grade 2. These were correctly classified as grade 3 with APTw imaging.
- Mean APT values can differentiate the tumour core from the tumour periphery.
- There is no statistically significant difference in the mean APT values between high grade gliomas and lymphomas.
Acknowledgements
No acknowledgement found.References
1. Togao O, Hiwatashi A, Yamashita K, Kikuchi K, Keupp J, Yoshimoto K, Kuga D, Yoneyama M, Suzuki SO, Iwaki T, Takahashi M, Iihara K, Honda H. Grading diffuse gliomas without intense contrast enhancement by amide proton transfer MR imaging: comparisons with diffusion- and perfusion-weighted imaging. EurRadiol. 2017 Feb;27(2):578-588
2. Bai Y, Lin Y, Zhang W, Kong L, Wang L, Zuo P, et al. Noninvasive amide proton transfer magnetic resonance imaging in evaluating the grading and cellularity of gliomas. Oncotarget. 2015 Jan 24; 8(4):5834– 42
3. Hilario A, Sepulveda JM, Perez-Nunez A et al (2014) A prognostic model based on preoperative MRI predicts overall survival in patients with diffuse gliomas. AJNR Am J Neuroradiol 35:1096–110
4. Jiang S, Yu H, Wang X, Lu S, Li Y, Feng L, Zhang Y, Heo HY, Lee DH, Zhou J, Wen Z. Molecular MRI differentiation between primary central nervous system lymphomas and high-grade gliomas using endogenous protein-based amide proton transfer MR imaging at 3 Tesla. EurRadiol. 2016 Jan;26(1):64-71.
5. Durmo F, Rydhog A, Testud F, Latt J, Schmitt B, Rydelius A, et al. (2020) Assessment of Amide proton transfer weighted (APTw) MRI for pre-surgical prediction of final diagnosis in gliomas. PLoS ONE 15(12): e0244003
6. Lingl, J.P.; Wunderlich, A.; Goerke, S.; Paech, D.; Ladd, M.E.; Liebig, P.; Pala, A.; Kim, S.Y.; Braun, M.; Schmitz, B.L.; et al. The Value of APTw CEST MRI in Routine Clinical Assessment of Human Brain Tumor Patients at 3T. Diagnostics 2022, 12, 490
Figures