2309
Intracranial arterial pulsatility assessed by 4D-flow imaging is associated with white matter degeneration
Linyun Xie1, Yao Zhang2, Hui Hong1, Shan Xu1, Lei Cui2, Luwei Hong2, Jixuan Li2, and Peiyu Huang1
1Department of Radiology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China, 2The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
1Department of Radiology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China, 2The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Synopsis
Keywords: Blood vessels, Velocity & Flow, Pulsatility
We investigate the association between the intracranial pulsatility assessed by 4D-flow imaging and white matter degeneration in a community cohort. White matter free water and tissue fractional anisotropy were used to reflect white matter degeneration. We found that the intracranial arterial pulsatility in different vessel segments was positively associated with increased free water, and negatively associated with tissue fractional anisotropy. After adjusting for age and vascular risk factors, the associations disappeared. Further investigations in larger samples are warranted.Introduction
The human brain is a blood-rich organ that is susceptible to pulsatile damage. 1Previous studies found that age-related stiffness in large arteries promotes the transmission of excess pulsatile energy into downstream vessels, which may cause brain damage. However, most studies focused on peripheral blood vessels such as the aorta. In this study, we aim to explore the association between intracranial arterial pulsatility in different vessel segments and white matter degeneration.Method
The study protocol has been approved by the ethics committee of the second affiliated hospital of Zhejiang University School of Medicine. A total of 65 elderly subjects were recruited from communities through advertisement. Each subject went through multi-modal MRI and full clinical assessments. Structural imaging, 4D-flow imaging, and diffusion tensor imaging were performed on a 3.0T scanner (uMR790, United Imaging, Shanghai). The parameters of 4D flow were: TR=30ms, TE=4.27ms, voxel size=0.7*0.7*2mm, flip angle=8, velocity encoding=100cm/s, scan time~10min. The parameters of DTI were: TR=8682ms, TE=75.8ms, field of view = 224 *224 mm, voxel size = 2*2*2mm, 70 axial slices, diffusion directions = 30, b = 0, 1000, 2000, and phase encoding direction = posterior-anterior. Another b0 image with an opposite phase encoding direction was acquired to correct EPI distortions.Vessel segmentation and flow analysis were performed in the cvi42 software (https://www.circlecvi.com/). Flow measuring planes were manually placed orthogonal to the course of the vessels in 13 vessel segments including the basilar artery, bilateral middle cerebral artery (MCA) M1 segment, bilateral internal carotid artery (ICA) C2, C4, C7 segment, bilateral anterior cerebral artery (ACA) A1 segment, and bilateral posterior cerebral artery (PCA) P1 segment. The contour of the vessel area was automatically segmented and manually adjusted. The flow PI was calculated using the equation: PI=(Qmax-Qmin)/Qmean; Q=flow rate; the Qmax and Qmin were defined within the cardiac cycle.
A bi-tensor model was used to analyze the white matter microstructure. We used Free water(FW) and FA-t to represent the white matter integrity. Image preprocessing was performed in Mrtrix3, including denoise, de-Gibbs, motion correction, and eddy-current correction. The mean white matter FW and tissue fractional anisotropy (FA-t) were calculated using the MarkVCID script (https://markvcid.partners.org/markvcid1-protocols-resources).2
First, we investigated the association between age, sex, vascular risk factors, and white matter diffusion measures using simple linear regression. Then, we explored the association between pulsatility and white matter degeneration. We set the FW or FA-t as the dependent variable, and the flow PI of all vessel segments as independent variables separately. Three models were adopted. Model 1 only included each flow PI as the independent variable. Model 2 was adjusted for age based on model 1. Model 3 was further adjusted for sex and vascular risk factors including hypertension, hyperlipidemia, diabetes mellitus, and smoking history. The p-value for statistical significance was set at 0.05, 2-tailed.
Result
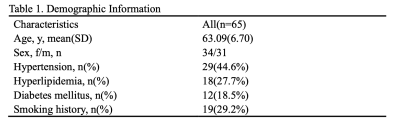
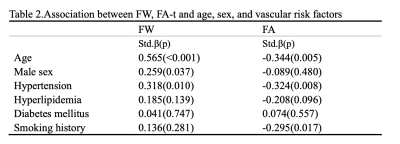
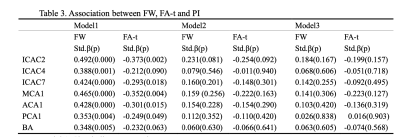
The demographic and vascular risk factor information of the subjects was shown in Table 1. A total of 65 subjects were included. The mean age of the subjects was 63.09, and 34 of them were females. In simple linear regression, age, male sex, and hypertension were associated with elevated FW. Meanwhile, age, hypertension, and smoking history were negatively associated with FA(Table 2).As shown in Table 3, the PIs of all vessel segments were positively associated with FW (Model 1). All PIs, except for ICAC4 and BA, were also negatively associated with the FA-t. Among all the vessel segments, the ICAC2 and MCA1 showed the strongest associations. After adjusting for age (Model 2), all the associations lost significance, but the association with ICAC2 was marginally significant. It further went away after including sex and vascular risk factors as covariates.
Discussion
We found that the intracranial arterial PI was associated with increased FW in all vessel segments, and negatively associated with FA-t in most vessel segmentations. Nevertheless, no significant association was found after adjusting for age. One possible reason is the relatively small sample size, which limited the detection of weak relationships. Another reason is that the subjects were relatively healthy and the SVD burden was low. Previously, Mailled et al. found that the aortic stiffness reflected by pulse wave velocity was associated with increased FW based on 2422 participants. 3 In our study, the association between ICAC2 PI with FW(p=0.081) and FA-t(p=0.092) almost reached significant, suggesting that intracranial arterial pulsatility may contribute more to brain white matter damages.Conclusion
The increased intracranial arterial pulsatility may play an important role in white matter degeneration.Further investigations in larger samples are warranted.Acknowledgements
NoneReferences
1. Mitchell, G. F. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985) 105, 1652–1660 (2008).
2. Huang, P. et al. White Matter Free Water is a Composite Marker of Cerebral Small Vessel Degeneration. Transl Stroke Res 13, 56–64 (2022).
3. Maillard, P. et al. Aortic Stiffness, Increased White Matter Free Water, and Altered Microstructural Integrity: A Continuum of Injury. Stroke 48, 1567–1573 (2017).
DOI: https://doi.org/10.58530/2023/2309