2304
Vascular architecture mapping reveals distinct microvascular profiles in healthy grey and white matter1Department of Neurology, Heidelberg University Hospital, Heidelberg, Germany, 2Department of Radiology, Heidelberg University Hospital, Heidelberg, Germany, 3Department of Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany, 4German Cancer Research Center, Heidelberg, Germany
Synopsis
Keywords: Blood vessels, Quantitative Imaging
Vessel architecture mapping (VAM) is a quantitative MR imaging technique based on dynamic changes in gradient-echo (R2*) and spin-echo (R2) relaxation rates during contrast agent administration, that can characterize cerebral blood vessel microstructure and their changes in vivo. We aimed to establish VAM parameter reference ranges in healthy brain tissue by scanning n=135 patients with whole-brain high resolution VAM at 3.0 Tesla MRI. Subsequent analysis revealed distinct microvascular profiles for cortical gray matter, supratentorial white matter and examined subcortical nuclei. These reference values may serve as a starting point for future VAM studies on cerebrovascular pathologies.Introduction
Cerebrovascular and neoplastic disease is typically linked to microvascular changes, e.g., microvascular proliferation in glioblastoma.1 Such changes can be monitored with vascular architecture mapping (VAM), a combined spin and gradient echo echo-planar imaging (SAGE-EPI) sequence that relies on dynamic changes in spin echo and gradient echo relaxation rates R2 and R2*, respectively, during contrast agent bolus passage.2 Plotting R2* versus R2 for every sampled time-point produces vortex curves, whose shape, slope, and direction depend on microvascular properties.3 While VAM has shown promise in identifying therapy response of antiangiogenic therapy in glioblastoma,3 or to differentiate glioma grades,4 there is a lack of reference ranges in healthy brain tissue, thus limiting interpretation and, therefore, application of VAM.Methods
VAM was performed for the whole brain of n=135 adult patients with at least two years stable suspected unilateral low-grade glioma, who had no prior history of chemo- or radiotherapy (mean age = 38.5 ±12.2 years, female/male = 66/69), at 3 Tesla (Prisma, Siemens) as previously described,5 using a multiband SAGE-EPI sequence with parallel imaging acceleration (parallel imaging factor = 3) and 20-channel head receive radio-frequency coil. Further sequence parameters were: TE (GE/SE) = 22/90ms, TR = 1.5s, multiband factor = 2, in-plane resolution = 2x2 mm2, slice thickness = 4.5mm, acquisition time = 90s. During administration of 0.1mmol/kg bodyweight gadoterate meglumine at a rate of 4ml/s and subsequent injection of a 20ml saline bolus, 60 SAGE-EPI readouts with 24 slices each were recorded. Before VAM analysis, motion correction was done with SPM 12.0 in Matlab R2020a, and changes in relaxation rates were determined as $$$ΔR(t)=-\frac{1}{TE} ln(\frac{S(t)}{S_0})$$$, for GE and SE signals, respectively. Contrast agent leakage effects were corrected for as in 6. Resulting vortex curves yielded the following parameters: vessel type indicator (VTI) as the signed area of the vortex curve, vascular inflow caliber (VIC) as its slope, and the length of the long and short axis of the vortex curve as SL and SA, respectively. Vessel size index (VSI) was calculated based on 2.Volume-of-interests (VOIs) were obtained on the healthy hemispheres contralateral to the suspected low-grade glioma, for cortical grey matter (cGM) and supratentorial white matter (WM) with FMRIB’s automated segmentation tool in FSL7 on isotropic T1-weighted images with resolution=(0.7mm)3, and co-registered to VAM parameter maps. VOIs for putamen (Put), globus pallidus (GP), thalamus (Thal), and caudate nucleus (CN) were identified using a digital human brain atlas in SPM (AAL3).8 Subsequent pair-wise post hoc comparisons between parameter distributions used Kruskal-Wallis tests with Dunn’s corrections.
Results
Cardiovascular risk factor prevalence in the participant cohort was similar to the Western European population: BMI 25.6 kg/m2 (± 4.0), arterial hypertension 15.6%, type 2 diabetes 3.7%, and smokers 24.4%. The obtained references ranges for all VAM parameters and VOIs can be found in Table 1. Figs. 1-2 show representative VAM parameter maps, indicating the contrasts between different brain regions. VAM parameter distributions differed significantly in all VAM parameters for cGM vs. WM, Thal, GP, and Put (all p<0.01). For VIC, VSI, SA, and SL, parameter distributions differed significantly between WM vs. Thal, GP, and CN (all p<0.01), but not for VTI. Remaining VOI pairs were not significantly different in VIC or VTI; specifically, no significantly different vascular profile was found between WM and Put, or between GM and CN, but distributions were significantly different in SA, SL, and VSI for WM vs. Put, and for SA and SL for GM vs. CN.Discussion
Healthy brain tissue showed an overall dominance of venules and capillary-like vessels, as indicated by negative values for VTI in all examined VOIs, and, compared to WM, cGM revealed larger vessel calibers (VIC, VSI), a dominance of venous vessels (VTI), and higher blood volume fractions (SL), in line with previous imaging studies.9,10 Distinct VAM profiles were found for cGM and subcortical grey matter regions such as GP and Thal, where microvessel density is typically high.11 Predominant vessel types (VTI) showed similarities with WM for GP and Thal, but indicated a more grey-matter like microvasculature profile in CN, potentially reflecting distinct embryological developments and functional specializations among the subcortical grey matter nuclei.12,13 Inter-subject variability in vessel size (VSI, VIC) was largest in CN, indicating increased vulnerability to age-dependent degeneration, as found previously ex vivo.14 Further work will need to explore dependencies on age and cerebrovascular risk factors.Conclusion
VAM in healthy adult brain reveals distinct microvascular profiles with similarities of vascular arrangements as described above. The obtained VAM parameter reference ranges may serve as starting point for future VAM investigations of cerebrovascular pathologies and may facilitate early discovery of microvascular changes in, e.g., neoplastic recurrence.Acknowledgements
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Contract Grant number: DFG ZI 1295/2-2) A. Hohmann was supported by the Rahel-Goitein-Straus-Program from the medical faculty of Heidelberg University.References
1. Das, S. & Marsden, P. A. Angiogenesis in Glioblastoma. N Engl J Med 369, 1561–1563 (2013).
2. Kiselev, V. G., Strecker, R., Ziyeh, S., Speck, O. & Hennig, J. Vessel size imaging in humans. Magn Reson Med 53, 553–563 (2005).
3. Emblem, K. E. et al. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med 19, 1178–1183 (2013).
4. Stadlbauer, A. et al. MR Imaging-derived Oxygen Metabolism and Neovascularization Characterization for Grading and IDH Gene Mutation Detection of Gliomas. Radiology 283, 799–809 (2017).
5. Zhang, K. et al. Vessel architecture imaging using multiband gradient-echo/spin-echo EPI. PLoS ONE 14, e0220939 (2019).
6. Boxerman, J. L., Schmainda, K. M. & Weisskoff, R. M. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27, 859–867 (2006).
7. Zhang, Y., Brady, M. & Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20, 45–57 (2001).
8. Rolls, E. T., Huang, C.-C., Lin, C.-P., Feng, J. & Joliot, M. Automated anatomical labelling atlas 3. Neuroimage 206, 116189 (2020).
9. Helenius, J. et al. Diffusion-weighted MR imaging in normal human brains in various age groups. AJNR Am J Neuroradiol 23, 194–199 (2002).
10. Bernier, M., Cunnane, S. C. & Whittingstall, K. The morphology of the human cerebrovascular system. Hum Brain Mapp 39, 4962–4975 (2018).
11. Kubíková, T., Kochová, P., Tomášek, P., Witter, K. & Tonar, Z. Numerical and length densities of microvessels in the human brain: Correlation with preferential orientation of microvessels in the cerebral cortex, subcortical grey matter and white matter, pons and cerebellum. J Chem Neuroanat 88, 22–32 (2018).
12. Wolfram-Gabel, R. & Maillot, C. Vascular networks of the nucleus lentiformis. Surg Radiol Anat 16, 373–377 (1994).
13. Wolfram-Gabel, R. & Maillot, C. [Vascular architecture of the caudate nucleus]. J Neuroradiol 24, 23–29 (1997).
14. Guan, J. et al. Vascular degeneration in Parkinson’s disease. Brain Pathol 23, 154–164 (2013).
Figures
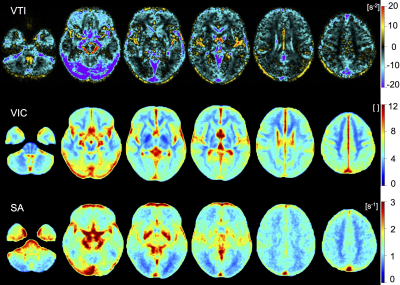
Figure 1: Axial representations of spatially normalized, averaged parametric maps (n=135) for parameters vessel type indicator (VTI), vascular inflow caliber (VIC) and short axis (SA).
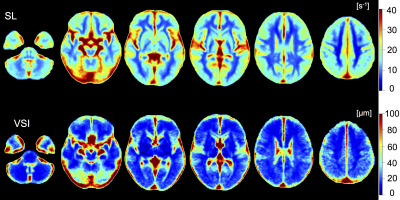
Figure 2: Axial representations of spatially normalized, averaged parametric maps (n=135) for parameters slope length (SL) and vessel size index (VSI).
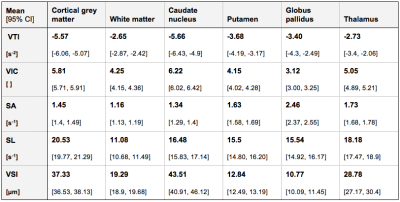
Table 1: Results from volume of interest analysis for 135 scans. Data are presented as mean values with 95% CI.