2301
Altered amide proton transfer weighted signal in different Suzuki grading and preinfarction period stages in patients with moyamoya disease1Functional and Molecular Imaging Key Laboratory of Sichuan Province, Department of Radiology, West China Hospital, Chengdu, China, 2Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu, China, 3Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu, China, 4Philips Healthcare, Xi'an, China
Synopsis
Keywords: Blood vessels, Vessels, moyamoya disease, amide proton transfer
To explore the alteration of amide proton transfer weighted (APTw) signal in different Suzuki stages and preinfarction period stages in patients with moyamoya disease (MMD), we enrolled 24 patients who underwent computed tomography perfusion (CTP), digital subtraction angiography (DSA), and APTw imaging. Although our results demonstrated that APTw values in the cerebral hemispheres of MMD patients with Suzuki stages V-VI and preinfarction period stage III are significantly higher than those in other stages, suggesting that the microenvironment of cerebral hemispheres in MMD patients with different Suzuki stages and preinfarction period stages suffers different severity of acidosis penumbra.Introduction
Moyamoya disease (MMD) is a chronic cerebrovascular disease characterized by stenosis or occlusion of the distal part of large intracranial arteries1. Suzuki grading is the gold standard grading criteria proposed by Suzuki2. With different Suzuki stages, the vascular network and related tissue show different microenvironments and metabolism. Besides, the perfusion status is evaluated by the preinfarction period stages3. Amide proton transfer weighted (APTw) imaging is a novel technique to detect endogenous dissociated peptides and proteins in tissue4. Besides, the internal pH environment can also affect APTw signal. Although previous studies have reported the possibility of pH-weighted APTw imaging in detecting acidosis penumbra in ischemic stroke5,6, few studies have reported how the APTw signal changes in patients with MMD. In this work, we use APTw imaging to investigate the alteration of APTw signal in MDD patients with different Suzuki and preinfarction period stages.Material and Methods
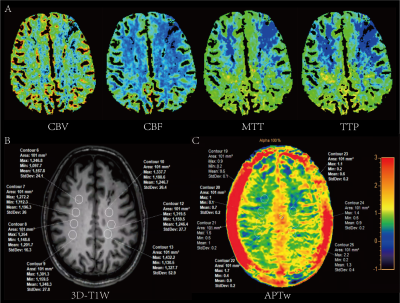
Twenty-four MMD patients (range 17-64 years; mean: 42 years, SD: 16) were enrolled. All patients underwent routine digital subtraction angiography (DSA) and computed tomography perfusion (CTP) examinations before magnetic resonance imaging (MRI). The angiographic features of DSA were used to evaluate the Suzuki stages. And we further divided the 13 patients into four subgroups according to the preinfarction period stages based on CTP3. All patients underwent MR examination on a 3.0T scanner (Ingenia Elition, Philips Healthcare, the Netherlands) with a 32-channel dedicated head coil. The structural images were acquired by using a high-resolution three-dimensional turbo field echo T1-weighed (3D-T1-TFE) sequence. The APTw images were acquired by using a 3D TSE-DIXON sequence. The criteria of region of interests (ROIs) placement were as follows: Firstly, ROIs were placed in cerebral hemispheres in the structural 3D-T1W images according to the site of impaired cerebral perfusion on CTP images. Multiple slices with multi-ROIs were applied if the extent of impaired cerebral perfusion was extensive. Secondly, the selected ROIs were copied from 3D-T1W to APTw images to ensure their locations were unchanged. Two experienced neuroradiologists, with 6 and 8 years of diagnosis experience, manually drew ROIs (95~105 pixels each) independently to obtain APTw values. The intraclass correlation coefficient (ICC) was performed to evaluate the inter-observer consistency of APTw values. The relationship between different Suzuki grading/preinfarction period stages and the corresponding APTw values were analyzed by ANOVA tests or Fisher’s Least Significant Difference (LSD) tests.Results
The ICC of the APTw value assessment of the two neuroradiologists was pretty good (ICC value > 0.85). The demographic and clinical characteristics of enrolled patients with MMD are shown in Table 1. According to the angiographic features on DSA, there were 17 (35.4), 23 (47.9), and 8 (16.7) cerebral hemispheres for the Suzuki stages of I-II, III-IV, and V-VI, respectively. Figure 1 showed multimodality images of a 36-year-old patient diagnosed with MMD and the related ROIs placement in the cerebral hemispheres. Table 2 and figure 2 showed that APTw_mean in patients with stages V-VI were significantly higher than those with stages I-II and stages III-IV (P=0.01 and P=0.03, respectively). Additionally, APTw_min in patients with different preinfarction period stages were significantly different (P=0.04), though there was no significant correlation between APTw_mean and preinfarction period stages in MMD patients (P=0.48) (Table 3). Further analysis showed that patients with preinfarction period stage III had higher APTw_min values than those with normal stage or preinfarction period stage II/IV (P=0.04, P=0.02, and P=0.04, respectively).Discussion
As can be seen from the results, APTw_mean in patients with Suzuki stages V-VI was significantly higher than those with stages I-II and III-IV. Although there was no statistical difference, we found a trend that the higher the Suzuki stages, the higher the APTw value. This finding suggested that with the aggravation of the stenosis of internal carotid arteries, APTw values of cerebral hemispheres is dynamically changing in a long-term chronic ischemic environment; that is, the acidosis of brain tissue will gradually alleviate during the dynamic progress of MMD. Our study also demonstrated that patients with preinfarction period stage III had higher APTw_min values than those with normal stage or preinfarction period stage II/IV. After the ischemia of brain tissue, the body activates the cerebral circulation reserve capacity to compensate. However, acidosis gradually worsens and reaches its peak at the preinfarction period stage III, as the cerebral circulation reserve is gradually depleted. Then ischemic brain tissue activates the cerebral metabolic reserve, partially alleviating ischemia and acidosis. In stage IV, APTw_min decreases, and cerebral tissue acidosis is further aggravated, suggesting that local brain tissue may develop into ischemic infarction if hypoperfusion cannot be effectively improved7. In the future, multi-center trials with a large sample size are required.Conclusion
The APTw values in the cerebral hemispheres of MMD patients with Suzuki stage V-VI and preinfarction period stage III are significantly higher than those in other stages, suggesting that the microenvironment of cerebral hemispheres in MMD patients with different Suzuki stages and preinfarction period stages suffers different severity of acidosis penumbra. And this dynamic change can be detected by APTw imaging technique, which might provide evidence for clinical correction of acid-base disturbance in brain tissue. Further studies with multi-center design and large sample size on the current topic are therefore recommended.Acknowledgements
This study was supported by grants from Natural Science Foundation of Sichuan Province, China (2022NSFSC1435) and the Fund of the Beijing Medical Award Foundation (YXJL-2022-0665-0189).References
1. Burke GM, et al. Moyamoya disease: A summary. Neurosurg Focus. 2009;26:E11.
2. Suzuki J, et al. Cerebrovascular Moyamoya Disease: Disease Showing Abnormal Net-Like Vessels in Base of Brain. Arch Neurol. 1969;20:288-99.
3. Yin H, et al. A Novel Staging System to Evaluate Cerebral Hypoperfusion in Patients With Moyamoya Disease. Stroke. 2018;49:2837-43.
4. Togao O, et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro Oncol. 2014;16:441-8.
5. Yu L, et al. Amide Proton Transfer MRI Signal as a Surrogate Biomarker of Ischemic Stroke Recovery in Patients with Supportive Treatment. Front Neurol. 2019;10:104.
6. Sun PZ, et al. Demonstration of magnetization transfer and relaxation normalized pH‐specific pulse‐amide proton transfer imaging in an animal model of acute stroke. Magn Reson Med. 2020;84:1526-33.
7. Wang E, et al. Mapping tissue pH in an experimental model of acute stroke - Determination of graded regional tissue pH changes with non-invasive quantitative amide proton transfer MRI. Neuroimage. 2019;191:610-7.
Figures