2300
Alterations of cerebrovascular reactivity and morphologies in de novo Parkinson's disease
Hongwei Li1, Xiali Shao2, Bingyi Wang1, Jian Wang2,3, Jia Jia4, Zhensen Chen1,5, He Wang1,5,6, and Lirong Jin4
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, China, 3Department of Radiology, Zhongshan Hospital, Fudan University (Xiamen Branch), Xiamen, China, 4Department of Neurology, Zhongshan Hospital, Fudan Universit, Shanghai, China, 5Key Laboratory of Computational Neuroscience and Brain-Inspired Intelligence (Fudan University), Ministry of Education, Shanghai, China, 6Human Phenome Institute, Fudan University, Shanghai, China
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, China, 3Department of Radiology, Zhongshan Hospital, Fudan University (Xiamen Branch), Xiamen, China, 4Department of Neurology, Zhongshan Hospital, Fudan Universit, Shanghai, China, 5Key Laboratory of Computational Neuroscience and Brain-Inspired Intelligence (Fudan University), Ministry of Education, Shanghai, China, 6Human Phenome Institute, Fudan University, Shanghai, China
Synopsis
Keywords: Blood vessels, Brain
Increasing evidence showed that vascular changes might be involved in Parkinson's disease. However, the altered markers of neurovascular unit and cerebral vessels that serve as blood flow adaptors, remain unclear in early stage of de novo PD. In this study, we aimed to investigate regional cerebral blood flow (CBF), cerebrovascular reactivity (CVR) and artery morphological changes in de novo PD patients including cognitive impairment (PD-MCI) and normal cognition (PD-NC). Our data revealed that in the patient group, CVR was significantly reduced in left superior occipital gyrus, whose feeding arteries were dilated, but there was no significant change in CBF.Introduction
Parkinson’s disease (PD) is a common progressive neurodegenerative disease. There is a growing appreciation that vascular alterations can contribute to disease onset and aggravate the neurodegenerative process1. Although cerebral blood flow (CBF) alternation has been reported in patients with PD, these results remain controversial. The discrepancy may be partly due to individual differences in recruited subjects, with varied disease duration and medication. In addition to CBF, other vascular related parameters including cerebrovascular reactivity (CVR) which is partly representative of neurovascular unit (NVU) integrity, and cerebrovascular morphologies are also important in reflecting vascular functions, however are rarely studied in the early stage of PD. Thus, the aim of this study is to investigate the patterns of CVR, CBF and cerebral arteries alterations in the early drug-naïve PD patients with different cognitive status using multimodal MRI.Materials and methods
SubjectsWe recruited 46 de novo PD patients and 49 age and gender matched healthy control subjects (HCs). PD-MCI (n=12) was diagnosed according to the level 2 criteria recommended by the MDS Task Force2. PD patients who did not meet the criteria for PD-MCI were classified as PD-NC (n=34).
Image acquisition
All MR data were acquired using a 3-Tesla MR unit (Discovery™ MR750, GE Healthcare, Milwaukee, WI). Resting-state BOLD images were acquired as follows: TR=2000ms,TE=30ms,flip angle=90°, FOV=24cm,matrix=64×64,number of slices=34,slice thickness=4.0mm,number of dynamic scans=210. ASL images were acquired using a 3D pCASL sequence: TR=4830ms;TE=10.5ms;labeling-duration=1500ms;post-labeling delay=2025ms;NEX=3;flip angle=155°. A 3D multi-echo GRE sequence was used to acquire QSM: TR=51.5ms;number of echoes=16;first-TE=2.9ms;TE-spacing=3ms;flip angle=12°; FOV=22cm;matrix = 220×220;acceleration factor=2.
Image processing
As shown in Figure-1A, the CBF maps were directly calculated according to PCASL one-compartment model3. The CVR and FC maps were generated from resting-state BOLD images. For CVR measures, after motion correction, smoothing, and linear detrending, the BOLD data were temporally filtered with a band-pass filter of 0.01 to 0.1Hz. And then the voxel-wise regression analysis was performed to generate a CVR index map, in which the whole brain signal was treated as an independent variable in this general linear model4. For FC analysis, the cluster results of one-way ANOVA in CVR, left superior occipital gyrus, were selected as the seed, and the FC was measured by a Pearson correlation between the seed and each voxel time courses.
Arterial morphology
The QSM magnitude images were used to measure arterial morphology. As shown in Figure-1B, vessel segmentation was performed on the first-echo magnitude image using self-developed routines based on a specialized software MeVisLab (Mevis, Germany). All segmentation results were visually checked and corrected manually by two neurologists who have over 5 years’ experience with neuroimaging interpretation. Finally, vessel centerlines and morphological parameters (Table-1), were extracted using Mimics Medical 21.0 software (Materialize NV, Leuven, Belgium).
Results
As shown in Figure-2A, one-way ANOVA revealed that the CVR of the left superior occipital gyrus (SOG) was significant different among three groups. The CVR in the same region of PD-MCI and PD-NC group were significantly lower than those in controls (Figure-2B), while no significant differences were found between PD-MCI and PD-NC group. Importantly, PD patients either with MCI or normal cognition showed no significant CBF differences than that of HCs. Even more, significant difference of functional connectivity between left SOG and cerebellum, right precuneus, right temporal inferior, right midcingulate, left inferior parietal and left precentral gyrus was showed among PD-MCI, PD-NC and HCs (Figure-3).As shown in Figure-4 and Table-1, the radius of RMCA-M1, LMCA-M1 and LPCA-P2 were significantly different among three groups, and there was a significant dilation in the patient groups. In addition, the arterial territory template was used to divide the territories of LPCA into three parts: proximal, middle and distal. The significant CVR differences were only identified in the territories of distal-LPCA, with a tendency to decrease in the PD-MCI and PD-NC group.
Discussion and Conclusion
In this study, we revealed that the CVR of left SOG in PD-MCI and PD-NC patients were significantly lower than that of the HCs, while no significant CBF changes were observed among these three groups, suggesting that microvascular dysfunction rather than perfusion defect might be involved in the early PD. The CVR alteration occurred prior to CBF changes, which can be served as an early biomarker of PD. In addition, FC changes between the left SOG and widespread brain regions were observed among the three groups. This finding implies that NVU disfunction may be partly induced by weakened neural circuits in PD. Furthermore, some arteries were dilated in PD-MCI and PD-NC compared to HCs, indicating an adaptive process to compensate for impaired microvascular function. Also of note, significant CVR differences were identified in the territories of distal-LPCA, which further confirmed the structure-function relationship. Finally, we pointed out that there were no significant changes in CBF, CVR and arterial radius between PD-MCI and PD-NC group. The disease, rather than cognitive status might be correlated with vascular changes in early PD. However, our results might be bias by the relatively small sample size. In the future, larger multicenter studies may help confirm the current findings. In conclusion, our results contribute to a better understanding of the relationship between NVU integrity, cerebral artery morphology and arterial territory perfusion in de novo early PD.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81971583), National Key R&D Program of China (No. 2018YFC1312900), Shanghai Natural Science Foundation (No. 20ZR1406400), Shanghai Municipal Science and Technology Major Project (No.2017SHZDZX01, No.2018SHZDZX01) and ZJLab, Young and Middle-aged Health Talents Training Project of Fujian Province (No. 2020GGB059).References
1. Paul, G. & Elabi, O.F. Microvascular Changes in Parkinson’s Disease- Focus on the Neurovascular Unit. Frontiers in Aging Neuroscience 14(2022).2. Litvan, I., et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Movement Disorders 27, 349-356 (2012).
3. Alsop, D.C., et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine 73, 102-116 (2015).
4. Liu, P., et al. Cerebrovascular reactivity mapping using intermittent breath modulation. NeuroImage 215, 116787 (2020).
Figures

Figure 1. Diagram of
post-processing steps. (A) CBF maps are acquired
using 3D PCASL; CVR and FC maps are generated based on resting-state BOLD images;
Artery morphological parameters are extracted from the first echo QSM
magnitude image. (B) Flowchart of semi-automated analysis of arterial
morphology, including vessel enhancement, segmentation,
arterial centerline extraction, morphological measurement.
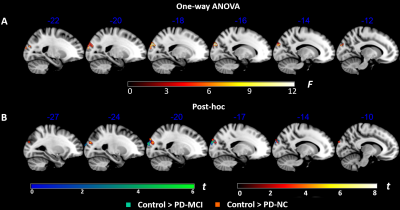
Figure 2. Comparisons of
regional CVR among the PD-MCI patients, PD-NC patients and normal controls. (A) The
significant CVR differences were identified in left superior occipital gyrus
among three groups. (B) Compared with control group, both PD-MCI and PD-NC
patients exhibited significant decreased CVR in this brain region,
but there was no difference between these two patients’ group. All results were
thresholded at p-FDR <0.05 cluster level.
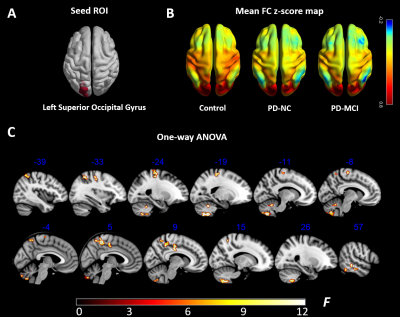
Figure 3. Functional connectivity comparison among the PD-MCI group, PD-NC
group and control group. (A) The results of one-way ANOVA in CVR, left
superior occipital gyrus, were selected as the seed. (B) Seed-to-voxel FC
z-score maps. (C) The brain regions with significant differences in FC relative
to the seed ROI were observed not only in cerebellum, temporal and parietal
lobe, but also in frontal lobe, like precentral gyrus. All results were thresholded at p-FDR <0.05 cluster level.
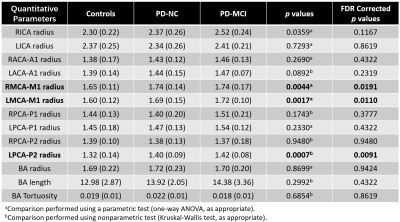
Table 1. Cerebrovascular Morphology Metrics. Significant statistical analysis results
are shown in bold. All data are mean ± SEM. Length and radius unit: mm; ICA:
internal carotid arteries; MCA: middle cerebral arteries; ACA: anterior
cerebral arteries; PCA: posterior cerebral arteries; BA: basilar arteries.
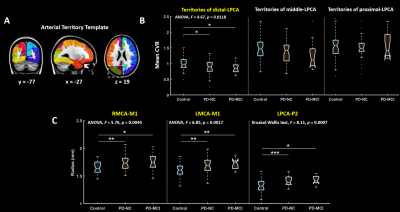
Figure 4. CVR in the territories of left-PCA, as well as
arterial morphological parameters, were compared among the three groups. Post-hoc LSD significance levels: *p<.05, **p<.01, ***p<.001. (A) Arterial territory template, divided based on
arterial transit time, covers the
territories of proximal, middle and distal PCA. (B) The significant CVR differences
were only identified in the territories of distal-LPCA, with a tendency to
decrease in the patients’ group. (C) Right MCA-M1, left MCA-M1, left PCA-P2, these three segments were obviously dilated compared
with control group.
DOI: https://doi.org/10.58530/2023/2300