2296
Effects of high-grade asymptomatic carotid artery stenosis on grey and white matter structure1Department of Neuroradiology, School of Medicine, Technical University of Munich, Munich, Germany, 2Philips GmbH Market DACH, Hamburg, Germany, 3Department for Vascular and Endovascular Surgery, School of Medicine, Technical University of Munich, Munich, Germany, 4Department of Neurology, School of Medicine, Technical University of Munich, Munich, Germany, 5Department of Diagnostic and Interventional Radiology, University Hospital Ulm, Ulm, Germany
Synopsis
Keywords: Blood vessels, Diffusion/other diffusion imaging techniques
Internal carotid artery stenosis (ICAS) is a known risk factor for stroke, additionally affecting brain structure and function. We investigated ICAS effects on white and grey matter structure using diffusion tensor imaging (DTI) and cortical thickness assessment, revealing alterations in various indices focused on the corpus callosum without signs of atrophy in grey matter. In addition, small vessel disease (SVD) burden, assessed by peak width of skeletonized mean diffusivity (PSMD), was increased in ICAS patients. Taken together, the structural alterations in white matter might precede those in grey matter, supporting the relevance of assessment of microstructure to detect early changes.Introduction
Internal carotid artery stenosis (ICAS) is a known risk factor for stroke,1 and can also affect cerebral hemodynamics2,3and cognition even in clinically asymptomatic patients.4,5 The pathogenesis of cognitive decline in ICAS patients, however, is not entirely understood, particularly the connection to structural alterations of white matter (WM) and grey matter (GM), which are independently linked to cognitive impairments.6,7 Some studies found a connection between WM hyperintensities (WMH) and ICAS,8,9 as well as between atrophy and stenosis,10,11 while others did not.12,13WMH are a frequent finding in cerebrovascular disease.7,14 There are several concepts for their development, including small vessel disease (SVD), which can affect the function of arterioles and capillaries.15,16 Numerous risk factors of SVD, such as hypertension and diabetes mellitus,15 are also present in patients with ICAS. The impact of SVD in patients with chronic hypoperfusion, however, is not entirely understood yet.
As pathophysiological processes commence prior to visibility of WMH on conventional T2-weighted MRI,17 parameters from advanced MRI targeting microstructural alterations, such as fractional anisotropy (FA), mean, axial, and radial diffusivity (MD, AD, RD) derived from diffusion tensor imaging (DTI), could be applicable to study early changes possibly leading to WMH.18,19 Moreover, influences of SVD could be investigated by peak width of skeletonized MD (PSMD), which was recently discussed as a promising biomarker for SVD.20 For investigation of GM, cortical thickness, derived from calculations of the distance between GM and WM surface,21 can be assessed.
Against this background, the aim of this study was to analyze effects of high-grade asymptomatic ICAS on GM and WM structure. Furthermore, to relate effects and extents of chronic hypoperfusion following ICAS on brain function, we also explored associations of brain microstructure with cognitive performance.
Methods
Twenty-eight patients (70.2±7.1y) with high-grade unilateral asymptomatic ICAS (>70% according to NASCET22) and 30 healthy age-matched volunteers (70.3±4.8y) were included in this prospective study (Table1). Participants underwent T1-weighted, fluid-attenuated inversion recovery (FLAIR), and DTI sequences on a 3T Philips Ingenia (Fig.1). Cognitive testing included Mini-Mental State Examination (MMSE)23 and Trail Making Test A and B (TMT-A and B).24The DTI data were processed using tract-based spatial statistics (TBSS) as implemented in FSL (v6.0.4).25 Within significant voxels (from comparing patients to volunteers) and by regions of interest using the JHU atlas,26 diffusion measures were extracted. Furthermore, whole-brain PSMD was calculated20 and compared between groups. Additionally, cortical thickness was calculated using CAT12 toolbox (v12.8)21 in SPM1227.
Two-sample t-tests were applied to compare whole-brain cortical thickness and DTI-derived parameters, the latter by applying 5,000 random permutations and threshold-free cluster enhancement (adjusted for age and sex). The WMH burden was visually graded according to Fazekas (from 0 to 3)29 and compared between patients and controls. Finally, Pearson’s correlation was used to evaluate the relationship between cognitive test results, diffusion measures, and PSMD.
Results
Whole-brain analysis of cortical thickness revealed no significant reductions in patients compared to controls (p=0.12). Fazekas scores did not differ significantly between patients and controls (p=0.38).However, DTI-derived indices in patients compared to healthy controls displayed significant increases of MD and AD (p<0.05/p<0.01, Fig.2). Significant voxels were found in both hemispheres, especially in deep WM and within the body, splenium, and genu of the corpus callosum (CC, Fig.2). Furthermore, PSMD was significantly elevated in patients compared to controls (p=0.012, Fig.3).
While patients performed similar to controls in MMSE (p=0.15) and TMT-A (p=0.68), patients were significantly slower in TMT-B (p=0.015). Correlation analysis of PSMD and MMSE results revealed a negative association, which was significant in patients (p=0.015, Fig.4). Additionally, declines in MMSE results correlated significantly with increasing AD in the genu and body of CC (p=0.022/p=0.038).
Discussion
Non-significant differences in cortical thickness or Fazekas scores between patients and controls could indicate that macrostructural alterations may not be severe in our cohort of asymptomatic ICAS patients. In contrast, significant differences in WM microstructure, in particular MD and AD increases, were found in both hemispheres. The locations of these microstructural alterations, focusing on the CC, could be connected to deteriorating cognitive performance,6,7 which was observed according to the patients’ TMT-B results in the present study. Notably, AD increases in the body and genu of the CC correlated with MMSE results, indicating that these WM regions might be especially vulnerable and could impact cognitive performance.A co-morbidity with SVD, which was likely manifested as an increased PSMD in patients, could further contribute to structural disintegration and cognitive decline. This is supported by our finding of decreasing MMSE results in patients with higher PSMD and the bilateral pattern of increased MD and AD. This might further suggest that microstructure might be affected globally by SVD, further endorsing degenerative alterations following ICAS.
Conclusion
We investigated the effects of ICAS on cortical thickness and WM microstructure and found that in clinically asymptomatic patients, WM was particularly affected as indicated by a higher SVD burden, while cortical thickness of patients did not significantly differ from an age-matched control group. Further, those microstructural alterations were associated with worse cognitive performance. These findings of microstructural alterations and their connection to cognition may support the use of diffusion-derived parameters as biomarkers of early disease progression.Acknowledgements
No acknowledgement found.References
1. D’Amore C, Paciaroni M. Border-zone and watershed infarctions. Frontiers of Neurology and Neuroscience 2012; 30: 181–184.
2. Powers WJ. The Effect of Hemodynamically Significant Carotid Artery Disease on the Hemodynamic Status of the Cerebral Circulation. Ann Intern Med 1987; 106: 27.
3. Kaczmarz S, Göttler J, Petr J, et al. Hemodynamic impairments within individual watershed areas in asymptomatic carotid artery stenosis by multimodal MRI. J Cereb Blood Flow Metab 2020; 380–396.
4. Lal BK, Dux MC, Sikdar S, et al. Asymptomatic carotid stenosis is associated with cognitive impairment. Journal of Vascular Surgery 2017; 66: 1083–1092.
5. Göttler J, Kaczmarz S, Nuttall R, et al. The stronger one-sided relative hypoperfusion, the more pronounced ipsilateral spatial attentional bias in patients with asymptomatic carotid stenosis. J Cereb Blood Flow Metab 2020; 40: 314–327.
6. Yamauchi H, Fukuyama H, Nagahama Y, et al. Atrophy of the Corpus Callosum Associated With Cognitive Impairment and Widespread Cortical Hypometabolism in Carotid Artery Occlusive Disease. Archives of Neurology 1996; 53: 1103–1109.
7. 2001–2011: A Decade of the LADIS (Leukoaraiosis And DISability) Study: What Have We Learned about White Matter Changes and Small-Vessel Disease? Cerebrovasc Dis 2011; 32: 577–588.
8. Chuang Y-M, Huang K-L, Chang Y-J, et al. Associations between Circle of Willis Morphology and White Matter Lesion Load in Subjects with Carotid Artery Stenosis. Eur Neurol 2011; 66: 136–144.
9. Ye H, Wang Y, Qiu J, et al. White matter hyperintensities and their subtypes in patients with carotid artery stenosis: a systematic review and meta-analysis. BMJ Open 2018; 8: e020830.
10. Muller M, van der Graaf Y, Algra A, et al. Carotid atherosclerosis and progression of brain atrophy: The SMART-MR Study. Annals of Neurology 2011; 70: 237–244.
11. Avelar WM, D’Abreu A, Coan AC, et al. Asymptomatic Carotid Stenosis is Associated with Gray and White Matter Damage. International Journal of Stroke 2015; 10: 1197–1203.
12. Streifler JY, Eliasziw M, Benavente OR, et al. Lack of Relationship Between Leukoaraiosis and Carotid Artery Disease. Archives of Neurology 1995; 52: 21–24.
13. Potter GM, Doubal FN, Jackson CA, et al. Lack of Association of White Matter Lesions with Ipsilateral Carotid Artery Stenosis. Cerebrovasc Dis 2012; 33: 378–384.
14. Purkayastha S, Fadar O, Mehregan A, et al. Impaired Cerebrovascular Hemodynamics are Associated with Cerebral White Matter Damage. J Cereb Blood Flow Metab 2014; 34: 228–234.
15. van Dijk EJ, Prins ND, Vrooman HA, et al. Progression of Cerebral Small Vessel Disease in Relation to Risk Factors and Cognitive Consequences: Rotterdam Scan Study. Stroke 2008; 39: 2712–2719.
16. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. The Lancet Neurology 2010; 9: 689–701.
17. de Groot M, Verhaaren BFJ, de Boer R, et al. Changes in Normal-Appearing White Matter Precede Development of White Matter Lesions. Stroke 2013; 44: 1037–1042.
18. Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007 Jul;4(3):316-29.
19. Le Bihan D, Johansen-Berg H. Diffusion MRI at 25: Exploring brain tissue structure and function. NeuroImage 2012; 61: 324–341.
20. Baykara E, Gesierich B, Adam R, et al. A Novel Imaging Marker for Small Vessel Disease Based on Skeletonization of White Matter Tracts and Diffusion Histograms: Novel SVD Imaging Marker. Ann Neurol 2016; 80: 581–592.
21. Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. NeuroImage 2013; 65: 336–348.
22. North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke 1991; 22: 711–720.
23. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975; 12: 189–198.
24. Gaudino EA, Geisler MW, Squires NK. Construct validity in the trail making test: What makes part B harder? Journal of Clinical and Experimental Neuropsychology 1995; 17: 529–535.
25. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage 2006; 31: 1487–1505.
26. Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 2008; 40: 570–582.
27. Statistical Parametric Mapping software (SPM12) Version 6225: www.fil.ion.ucl.ac.uk/spm.
28. MATLAB and Statistics Toolbox Release 2016b, The MathWorks, Inc., Natick, Massachusetts, United States.
29. Fazekas F, Chawluk J, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. American Journal of Roentgenology 1987; 149: 351–356.
Figures
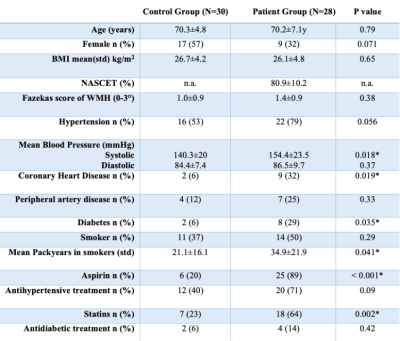
Table 1 - Demographics
Demographic data of patients and controls. BMI = body mass index, n.a. = not applicable, WMH = white matter hyperintensities. Unpaired two-sided t-tests for age, BMI, packyears, blood pressure, Chi2 test for the remaining parameters.
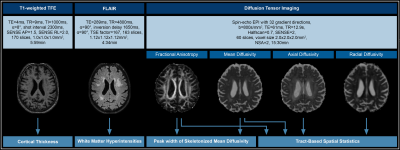
Figure 1. MRI acquisition and Processing
Cortical thickness was calculated from T1-weighted turbo-field echo (TFE) images via CAT 1221 and SPM 1227. Hyperintensities were graded based on the Fazekas score using fluid-attenuated inversion recovery (FLAIR) imaging.29Diffusion tensor data were processed using FSL tract-based spatial statistics (TBSS)25 for group tests, using 5,000 random permutations and applying threshold-free cluster enhancement adjusted for age and sex. Peak width of skeletonized mean diffusivity (PSMD) was calculated from preprocessed FA and MD.
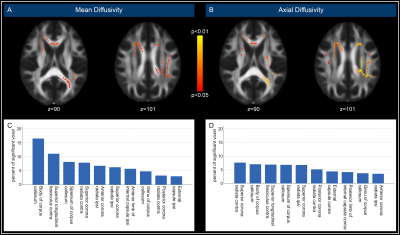
Figure 2. Spatial distribution of DTI results
Color overlay highlights voxels with significantly increased mean diffusivity (MD; A) and axial diffusivity (AD; B) in patients compared to controls in both hemispheres. Analysis of the spatial distribution of significant voxels (C, D) revealed a focus on the corpus callosum (CC), notably for MD (C). To compare the ipsilateral and contralateral hemispheres, maps of patients with left-sided stenosis were flipped. Contra/ipsi = contralateral/ipsilateral hemisphere to stenosis.
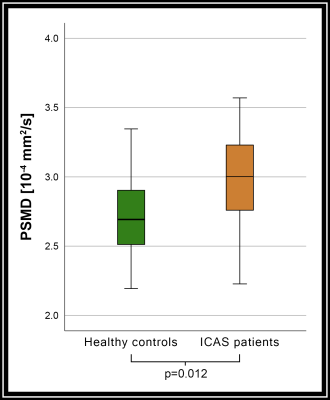
Figure 3. PSMD in healthy controls and ICAS patients.
Peak width of skeletonized mean diffusivity (PSMD) was significantly higher (p<0.05) in patients with ICAS than in age matched healthy controls, indicating an influence of small vessel disease (SVD) on white matter (WM) microstructure.
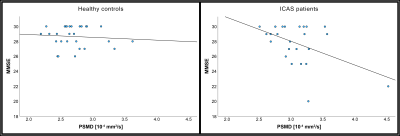
Figure 4. Scatter plots of PSMD and MMSE results.
While Mini-Mental State Examination (MMSE) scores did not differ significantly between ICAS patients (left) and controls (right), MMSE scores in patients correlated significantly with elevated PSMD (p=0.015), indicating that pronounced small vessel disease (SVD) might be connected to declines in cognitive performance.