2291
Diffuse destruction of white matter myelin sheath integrity associated with cognitive impairment of long-term Parkinson’s disease patients
Bingbing Gao1, Liangjie Lin2, and Yanwei Miao1
1the First Affiliated hospital of Dalian Medical university, Dalian, China, 2Clinical and Technical Support, Philips Healthcare, Beijing, China
1the First Affiliated hospital of Dalian Medical university, Dalian, China, 2Clinical and Technical Support, Philips Healthcare, Beijing, China
Synopsis
Keywords: Parkinson's Disease, Dementia
The incidence of Parkinson's disease patients combined with dementia increased significantly with the extension of disease course, and increased from 26.3% to 83% at twenty years after diagnosis. This study aims to use diffusion tensor imaging to detect the pattern of white matter damage in long-term PD patients with cognitive impairment, and results indicated that PD patients have diffuse FA decrease of white matter that is correlated with cognitive status.Introduction
Cognitive impairment is one of the major non-motor syndromes in PD, while the neuropathological basis of dementia in PD is still not clear. Neuroimaging studies can help better understand the pathophysiology and symptoms of Parkinson's disease (PD). Multiple studies have utilized data driven diffusion tensor imaging (DTI) analyses to explore the white matter structure alteration in PD patients with early stage of cognitive impairment in comparison to control subjects with normal cognition. As reported, the characteristic white matter abnormalities associated with cognitive impairment in PD is that the diffusion abnormalities are predominantly shown as the diffusivity changes but not the FA [1]. Considering DTI as a promising marker for monitoring cognitive impairment progressing of PD patients, further DTI studies of PD clinical trials are needed. This study aims to detect the white matter damage pattern of long-term PD patients and its association with cognitive impairment based on data driven DTI analysis.Material and method
This study was approved by the local ethics committee of the First Affiliated Hospital of Dalian Medical University. Informed consents were obtained from patients or legal guardians before the study. 72 PD patients were prospectively enrolled. PD diagnosis was confirmed by neurologist according to Chinese guidelines for the Treatment of Parkinson’s disease (fourth edition) formulated by the Movement Disorders and Parkinson's Disease Group of the Neurology Branch of the Chinese Medical Association in 2020 [2]. The guidelines also included the exclusion criteria of PD. 51 sex- and age- matched control subjects with normal cognition (CN group) were recruited in this study. The exclusion criteria for those in the HC group were history of surgery, cardiovascular disease, hypertension, diabetes, and other chronic diseases. All participants were right-handed. Montreal Cognitive Assessment (MoCA) were applied in all participant. DTI images were obtained with a 3.0T MRI scanner (Ingenia CX, Philips Healthcare, Best, the Netherlands) equipped with a 32-channel phase-array head coil, using a single-shot echo-planar imaging (SS-EPI) sequence. The parameters were detailed in Table 1. DTI data were preprocessed using the Functional MRI of the Brain (FMRIB) Software Library (FSL) version 5.0.9 to get the FA, MD map for statistical analysis. All images were visually inspected after each step. Voxel-wise statistical analyses of the FA, MD maps between the two groups were performed using Trace-based spatial statistics (TBSS) toolbox in FSL. The relationship between DTI parameters and neurocognitive assessments were also analyzed. The intergroup comparison was performed using independent sample t-test with age, sex and years of education as covariates. Results were reported at the P < 0.05 level after 5,000 permutations, as well as threshold-free cluster enhancement (TFCE) for multiple comparisons. By FSL's Cluster, statistically significant clusters of the results were identified with two white matter templates: The John Hopkins University (JHU) -International Consortium of Brain Mapping DTI-81 WM labels and JHU White-Matter Tractography Atlas template. The correlation between DTI parameters and neurocognitive assessments were analyzed using Spearman correlation coefficient. Statistical significance was defined as two-tailed P < 0.05.Results
Compared to the CN group, the FA value show diffuse decrease (p=0.002, TFCE-corrected) in 16 independent clusters (vox > 200) in the PD group (Figure 1). Two of the significant clusters were detailed in Table 2. MD value had no significant different between two groups. The correlation between cluster FA value and MoCA was observed to be significantly positive (Figure 2).Conclusion
PD patients with cognitive impairment show diffuse FA decrease of white matter, and this is correlated with cognitive status. DTI combined with neurocognitive tests may be a valuable biomarker for identifying cognitive impairments in PD.Discussion
FA measures the directionality of randomized water molecular motion, decreased FA represented the break of nerve fiber arrangements, axonal integrity, and demyelination. MD measures the magnitude of water diffusion. A high MD is thought to indicate broad cellular damages including edema and necrosis. The diffuse decreased FA suggests that the white matter integrity was extensively damaged in PD patients with cognitive impairment, and it is connected to the patients’ cognitive status. Meanwhile, the MD value shows no significant difference may imply there is no neuron edema in the late disease. The cognitive impairment spectrum of PD consists of executive function, language, attention, memory, and visuospatial skills. The previous study has revealed different patterns of white matter diffusivity underlie impairments in distinct cognitive domains of PD patients [3]. Results by our study may show the unique pattern of white matter diffusivity in late PD. More in-depth and detailed research is needed.Acknowledgements
No acknowledgement found.References
[1] Zhang Y, Burock MA. Diffusion Tensor Imaging in Parkinson's Disease and Parkinsonian Syndrome: A Systematic Review. Front Neurol. 2020, 11:531993. doi:10.3389/fneur. [2] the Movement Disorders and Parkinson's Disease Group of the Neurology Branch of the Chinese Medical Association. Chinese guidelines for the Treatment of Parkinson’s disease (fourth edition) [J]. Chinese Journal of Neurology,2020,53(12):973-986. DOI: 10.3760/cma.j.cn113694-20200331-00233. [3] Zheng Z, Shemmassian S, Wijekoon C, Kim W, Bookheimer SY, Pouratian N. DTI correlates of distinct cognitive impairments in Parkinson's disease. Hum Brain Mapp. 2014;35(4):1325-1333. doi:10.1002/hbm.22256Figures
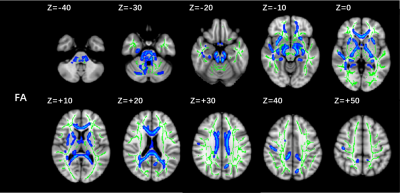
Figure 1 Post hoc
analyses result between PD patients and CNs. Green represents the mean FA
skeleton of all subjects. Blue represents regions with significant statistical
values (p < 0.05, TFCE-corrected). PD, Parkinson’s disease; CN,
healthy control with normal cognitive; FA, fractional anisotropy.
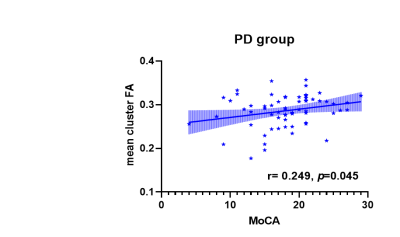
Figure 2 Relationship
between mean cluster FA and MoCA of PD group. PD, Parkinson’s disease; MoCA, Montreal
Cognitive Assessment; FA, fractional anisotropy.
DOI: https://doi.org/10.58530/2023/2291