2288
Disrupted Brain Structural Network Connection in de novo Parkinson’s Disease with Rapid Eye Movement Sleep Behavior Disorder1Guangzhou First People's Hospital, Guangzhou, China, 2Guangzhou Women and Children's Medical Center, Guangzhou, China, 3The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China, 4Philips Healthcare, Guangzhou, China
Synopsis
Keywords: Parkinson's Disease, White Matter, White matter connectivity, rapid movement sleep behavior disorder
To investigate the changes of white matter structural network connectivity in PD patients with probably rapid movement sleep behavior disorder (PD-pRBD). This study included PD-pRBD (n = 74) and PD with no probably RBD (PD-npRBD)(n = 97) and healthy contral (HC, n=73). The results showed that compared with the PD-npRBD group, the nodal efficiency (Ne) of the right insula and left middle frontal gyrus increased, while the Ne of the left temporal pole decreased. We conclude that changes in the right insula, left temporal pole and left middle frontal gyrus Ne may play key roles in the pathogenesis of PD-RBD.Introduction
One of the most common non-motor symptoms of Parkinson's disease (PD) is rapid eye movement sleep (REM) behavior disorder (RBD), which is characterized by dream-related motor behavior and accompanied by phasic tension electromyography during REM sleep. Evidence indicates that RBD in PD patients is associated with greater motor and nonmotor symptoms, such as smell disorders, constipation, visual hallucinations, cognitive impairment, depression, and impulse control disorders[1]. However, the potential neurological mechanisms underlying RBD in such patients are yet to be elucidated. In recent years, the white matter (WM) structural network connectome constructed by diffusion tensor imaging (DTI) has been increasingly analyzed with graph theory approaches, especially in PD patients, to determine alterations in the topological properties of brain structures[2, 3]. However, it remains unclear whether PD patients with probably rapid movement sleep behavior disorder (PD-pRBD) and PD patients with no probably rapid movement sleep behavior disorder (PD-pRBD) patients differ inWM connectivity. Therefore, this study aimed to explore alterations in the WM structural network connectome in PD-pRBD patients and assess whether REM sleep behavior disorder screening questionnaire (RBDSQ) score correlate with structural topological network changes, which might provide deeper insight into the neuropathological mechanisms underlying PD-RBD.Methods
This study included 171 de novo PD patients and 73 healthy controls (HC) recruited from the Parkinson’s Progression Markers Initiative (PPMI)database. The patients were divided into two groups, PD with probable RBD (PD-pRBD, n = 74) and PD without probable RBD (PD-npRBD, n = 97), according to the RBDSQ score. Individual structural networks of the brain were constructed based on deterministic fiber tracking and analyses were performed using graph theory. Differences in global and nodal topological properties were analyzed among the three groups. After that, post hoc analyses were performed to explore further differences. Finally, correlations between significantly different properties and RBDSQ scores were analyzed in the PD-pRBD group.Results
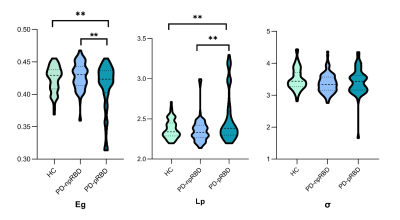
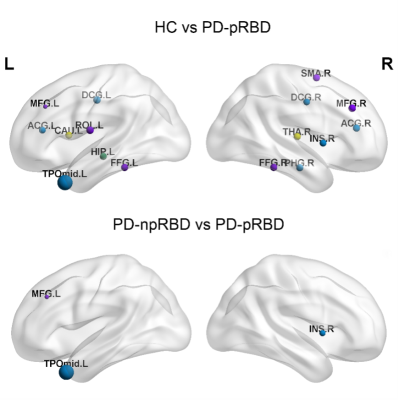
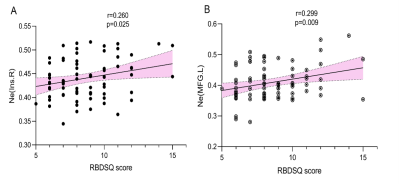
All three groups presented a small-world organization. PD-pRBD patients exhibited diminished global efficiency(t=3.480; p=0.002) and increased shortest path length (t=-3.099; p=0.003) compared with PD-npRBD patients and HCs. (FIG. 1) In nodal property analyses, compared with HCs, the brain regions of the PD-pRBD group with changed nodal efficiency (Ne) were widely distributed, mainly in neocortical and paralimbic regions. While compared with the PD-npRBD group, only increased Ne in the right insula, left middle frontal gyrus, and decreased Ne in the left temporal pole were discovered. (FIG.2) In addition, RBDSQ score was positively correlated with right insular Ne (r=0.260, p=0.025) and left middle frontal gyrus Ne (r=0.299, p=0.009) in PD-pRBD patients. (FIG.3)Discussion
In this study, PD-pRBD patients showed extensive alterations in Ne when compared with HCs. The nodes were widely distributed in the neocortex and paralimbic system. These findings suggest that the pathophysiology of RBD in PD not only involves midbrain dysfunction but also a wide range of cerebral cortex abnormalities, whose functional and structural abnormalities may be associated with the motor and cognitive-emotional dysfunction of PD-RBD. When compared with the PD-npRBD group, altered Ne in PD-pRBD patients was distributed only in the right insula, left temporal pole, and left middle frontal gyrus. Also, a positive correlation was discovered in our results between the Ne in the right insula and left middle frontal gyrus with RBDSQ scores. The insular and temporal poles are considered part of the paralimbic system. The insula receives information from sensory pathways through the thalamus, integrates information about the state of the body, and sends output signals to other limbic-related structures, such as the amygdala and striatum[4]. The temporal pole is associated with high-level cognitive processes: visual processing of complex objects, semantic processing in all modalities, and socio-emotional processing[5]. The behaviors observed in RBD are violent, and the associated dreams are associated with feelings of sadness and fear. Thus, we speculated that the paralimbic cortex related to emotional control was involved in the pathogenesis of PD-RBD[6].Conclusion
PD-pRBD patients showed disrupted topological organization of white matter in the whole brain.Acknowledgements
No acknowledgement found.References
[1] St Louis EK, Boeve BF. REM Sleep Behavior Disorder: Diagnosis, Clinical Implications, and Future Directions. Mayo Clin Proc. 2017. 92(11): 1723-1736.
[2] Li C, Huang B, Zhang R, et al. Impaired topological architecture of brain structural networks in idiopathic Parkinson's disease: a DTI study. Brain Imaging Behav. 2017. 11(1): 113-128.
[3] Nigro S, Riccelli R, Passamonti L, et al. Characterizing structural neural networks in de novo Parkinson disease patients using diffusion tensor imaging. Hum Brain Mapp. 2016. 37(12): 4500-4510.
[4] Furl N, Averbeck BB. Parietal cortex and insula relate to evidence seeking relevant to reward-related decisions. J Neurosci. 2011. 31(48): 17572-82.
[5] Herlin B, Navarro V, Dupont S. The temporal pole: From anatomy to function-A literature appraisal. J Chem Neuroanat. 2021. 113: 101925.
[6] Valli M, Cho SS, Masellis M, et al. Extra-striatal dopamine in Parkinson's disease with rapid eye movement sleep behavior disorder. J Neurosci Res. 2021. 99(4): 1177-1187.
Figures