2283
Abnormal intrinsic neural timescale in Parkinson’s disease
Yarui Wei1, Chunyan Zhang2, Yuanyuan Peng2, Chen Chen 2, Shaoqiang Han2, Weijian Wang2, Yong Zhang2, Hong Lu3, and Jingliang Cheng2
1Department of Magnetic Resonance Imaging, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 2Department of Magnetic Resonance Imaging, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 3Department of Neurology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
1Department of Magnetic Resonance Imaging, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 2Department of Magnetic Resonance Imaging, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 3Department of Neurology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Synopsis
Keywords: Parkinson's Disease, fMRI (resting state)
It’s unclear whether abnormal neural information stored and different temporal feature at different stages in Parkinson’s disease (PD). Here, we estimated the intrinsic timescales using the magnitude of the autocorrelation of intrinsic neural signals by resting state functional magnetic resonance imaging data in 74 PD patients, including 44 patients in the early stage and 30 patients in the late stage. Our findings suggest that PD patients exhibit abnormal intrinsic timescales in visual, sensorimotor, and cognitive systems, and at different stages, distinct patterns of intrinsic timescales in cerebral cortex, which might provide new insights for the neural substrate of PD.Background or Purpose
Specialization and hierarchy are organizing principles for primate cortex, and cortical areas are also specialized in the temporal domain. The neural timescale in a local brain area reflects the function of that area in many trials of a task1,2 or even in the absence of direct stimulus processing3,4. Moreover, the intrinsic neural timescales at resting state could predict the neural activity of the brain region in a task5-7, and the sensory and prefrontal areas exhibit shorter and longer timescales at resting state, respectively3. Parkinson’s disease (PD) is a progressive neurological disorder characterized by motor (tremor, rigidity, and slowness of movements and abnormal sensorimotor integration)8,9 and nonmotor (visual dysfunction and cognitive impairment)10-13 symptoms. Resting state fMRI studies also showed abnormal neural activity or functional connection in motor14-17 and nonmotor18-21 areas. However, whether abnormal neural information stored in sensorimotor, visual, and cognitive-related areas was also unclear. Because PD is a progressive neurodegenerative disease22,23, we also explored the differences of intrinsic timescales among patients with PD in the early stage (PD-ES) and the late stage (PD-LS) and healthy controls (HC).Methods
The present study estimated the intrinsic timescales by using the magnitude of the autocorrelation of intrinsic neural signals in 74 PD patients. PD patients were assessed the severity of motor symptoms and cognitive impairments by using the unified Parkinson's disease rating scale (UPDRS), a revised severity classification Hoehn and Yahr (HY) scale, Montreal cognitive assessment (MoCA), and mini-mental state examination (MMSE). Based on HY scale, 74 patients with PD were split into 44 patients with PD-ES (the scores of the HY scale were ≤2) and 30 patients with PD-LS (the scores of the HY scale were >2). To investigate the correlations among abnormal intrinsic timescale, symptom severity, age, and structural images, we calculated the Spearman rank correlation coefficients for clinical measures, age, and grey matter volume (GMV) with significant results between groups.Results
We identified that the PD group had shorter intrinsic timescales in the bilateral lingual gyri, bilateral postcentral gyri, and right middle cingulum gyrus, and longer timescale in the right middle frontal gyrus compared with the HC group (Figure 1). The intrinsic timescale of the bilateral lingual gyri in the PD group negatively correlated with the scores of the HY scale (Figure 2). Moreover, the intrinsic timescales in bilateral lingual gyri, the right postcentral gyrus, and the right middle cingulum gyrus negatively correlated the age of the PD group, respectively (Figure 3, upper). And we found that the age in the PD group negatively correlated with the scores of MoCA and MMSE (Figure 3, lower). We also found positively significant correlation between the intrinsic timescale and the GMV in the right postcentral gyrus (Figure 4). The one-sample ANOVA revealed significant between-group differences in intrinsic timescales of bilateral lingual gyri, the left precuneus, and the right middle cingulum gyrus (Figure 5, upper). Post hoc comparisons using Bonferroni’s test showed that the PD-LS group had shorter timescale in the left precuneus than that in the PD-ES group and shorter timescales in bilateral calcarine and lingual gyri than that in the HC group (Figure 5, lower). Increasingly, longer timescales in the left superior frontal gyrus, the left inferior frontal gyrus, the right middle frontal gyrus were also found in the PD-ES group than the HC group (Figure 5, lower).Discussion
In this study, we explored the intrinsic timescales, which relates to the functional hierarchy of the brain, in PD patients. Our findings showed abnormal temporal property of local neural activity in the visual, sensorimotor, and cognitive systems in PD, and the previous studies also found it through other neuroimaging methods24-29. Increasingly, the PD-ES group had longer timescales in the anterior cortical regions, whereas the PD-LS group had shorter timescales in the posterior cortical regions, which might associate with cognitive impairment. The cognitive impairment of the PD-LS group was more severity than that of the PD-ES group. Distinct patterns of local cerebral glucose metabolism were also found in PD with and without mild cognitive impairment (PDNC and PDMCI): the PDNC patients had limited areas of hypometabolism in the frontal and occipital cortices, and the PDMCI patients had extensive areas of hypometabolism in the posterior cortical regions, including the temporo-parieto-occipital junction, medial parietal, and inferior temporal cortices30. Hirano et al.31 suggested that the frontal cortex was associated with impulse control disorders and that posterior brain areas were related to cognitive decline in PD. It is reasonable that significant correlations between cognitive impairments and the age were found in PD, because PD is one of the most common age-related neurodegenerative disorders32. Furthermore, our findings suggest that the GMV and intrinsic timescales might be independent and complementary measures for PD.Conclution
Our findings suggest that PD patients exhibit abnormal intrinsic timescales in visual, sensorimotor, and cognitive systems, which provide new insights for the neural substrate of PD. Distinct patterns of intrinsic timescales between the PD-ES and PD-LS groups also indicate that intrinsic timescale may be a new neuroimaging biomarker across disease stage in PD, which have the potential to improve clinical care and management.Acknowledgements
This research study was supported by the National Natural Science Foundation of China (81601467, 81871327), Key Scientific Research Projects of Henan Provincial Colleges and Universities (23A320004), Medical Science and Technology Co-construction Project of Henan Province (LHGJ20220404), and Industrial Technology Foundation Public Service Platform Project of China (CEIEC-2020-ZM02-0103/03).References
1. Hasson U, Yang E, Vallines I, et al. A hierarchy of temporal receptive windows in human cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(10):2539-50. 2. Honey CJ, Thesen T, Donner TH, et al. Slow cortical dynamics and the accumulation of information over long timescales. Neuron. 2012;76(2):423-434. 3. Murray JD, Bernacchia A, Freedman DJ, et al. A hierarchy of intrinsic timescales across primate cortex. Nature neuroscience. 2014;17(12):1661-3. 4. Wengler K, Goldberg AT, Chahine G, et al. Distinct hierarchical alterations of intrinsic neural timescales account for different manifestations of psychosis. eLife. 2020;9:56151. 5. Cirillo R, Fascianelli V, Ferrucci L, et al. Neural Intrinsic Timescales in the Macaque Dorsal Premotor Cortex Predict the Strength of Spatial Response Coding. iScience. 2018;10:203-210. 6. Golesorkhi M, Gomez-Pilar J, Tumati S, et al. Temporal hierarchy of intrinsic neural timescales converges with spatial core-periphery organization. Communications biology. 2021;4(1):277. 7. Wolff A, Berberian N, Golesorkhi M, et al. Intrinsic neural timescales: temporal integration and segregation. Trends in cognitive sciences. 2022;26(2):159-173. 8. Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Movement disorders : official journal of the Movement Disorder Society. 2003;18(3):231-240. 9. Lewis GN, Byblow WD. Altered sensorimotor integration in Parkinson's disease. Brain : a journal of neurology. 2002;125(Pt 9):2089-99. 10. Weil RS, Schrag AE, Warren JD, et al. Visual dysfunction in Parkinson's disease. Brain : a journal of neurology. 2016;139(11):2827-2843. 11. Ciccarelli N, Anzuino I, Pepe F, et al. The facial emotion recognition deficit in Parkinson's disease: Implications of a visual scanning strategy. Neuropsychology. 2022;36(4):279-287. 12. Litvan I, Aarsland D, Adler CH, et al. MDS Task Force on mild cognitive impairment in Parkinson's disease: critical review of PD-MCI. Movement disorders : official journal of the Movement Disorder Society. 2011;26(10):1814-24. 13. Petrou M, Dwamena BA, Foerster BR, et al. Amyloid deposition in Parkinson's disease and cognitive impairment: a systematic review. Movement disorders : official journal of the Movement Disorder Society. 2015;30(7):928-35. 14. Bell PT, Gilat M, O'Callaghan C, et al. Dopaminergic basis for impairments in functional connectivity across subdivisions of the striatum in Parkinson's disease. Human brain mapping. 2015;36(4):1278-91. 15. Manza P, Zhang S, Li CS, et al. Resting-state functional connectivity of the striatum in early-stage Parkinson's disease: Cognitive decline and motor symptomatology. Human brain mapping. 2016;37(2):648-62. 16. Zang Z, Song T, Li J, et al. Simultaneous PET/fMRI revealed increased motor area input to subthalamic nucleus in Parkinson's disease. Cerebral cortex. 2022;Advance online publication 17. Li J, Liao H, Wang T, et al. Alterations of Regional Homogeneity in the Mild and Moderate Stages of Parkinson's Disease. Frontiers in aging neuroscience. 2021;13:676899. 18. Ruppert MC, Greuel A, Freigang J, et al. The default mode network and cognition in Parkinson's disease: A multimodal resting-state network approach. Human brain mapping. 2021;42(8):2623-2641. 19. Baggio HC, Sala-Llonch R, Segura B, et al. Functional brain networks and cognitive deficits in Parkinson's disease. Human brain mapping. 2014;35(9):4620-34. 20. Sun HH, Pan PL, Hu JB, et al. Alterations of regional homogeneity in Parkinson's disease with "pure" apathy: A resting-state fMRI study. Journal of affective disorders. 2020;274:792-798. 21. Lopes R, Delmaire C, Defebvre L, et al. Cognitive phenotypes in parkinson's disease differ in terms of brain-network organization and connectivity. Human brain mapping. 2017;38(3):1604-1621. 22. Mitchell T, Lehericy S, Chiu SY, et al. Emerging Neuroimaging Biomarkers Across Disease Stage in Parkinson Disease: A Review. JAMA neurology. 2021;78(10):1262-1272. 23. Filippi M, Basaia S, Sarasso E, et al. Longitudinal brain connectivity changes and clinical evolution in Parkinson's disease. Molecular psychiatry. 2021;26(9):5429-5440. 24. Shang S, Ye J, Wu J, et al. Early disturbance of dynamic synchronization and neurovascular coupling in cognitively normal Parkinson's disease. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2022;42(9):1719-1731. 25. Bellot E, Kauffmann L, Coizet V, et al. Effective connectivity in subcortical visual structures in de novo Patients with Parkinson's Disease. NeuroImage Clinical. 2022;33:102906. 26. Nackaerts E, Nieuwboer A, Broeder S, et al. Altered effective connectivity contributes to micrographia in patients with Parkinson's disease and freezing of gait. Journal of neurology. 2018;265(2):336-347. 27. Tian Y, Chen HB, Ma XX, et al. Aberrant Volume-Wise and Voxel-Wise Concordance Among Dynamic Intrinsic Brain Activity Indices in Parkinson's Disease: A Resting-State fMRI Study. Frontiers in aging neuroscience. 2022;14:814893. 28. Cai W, Young CB, Yuan R, et al. Dopaminergic medication normalizes aberrant cognitive control circuit signalling in Parkinson's disease. Brain : a journal of neurology. 2022;Advance online publication 29. Xu J, Yu M, Wang H, et al. Altered Dynamic Functional Connectivity in de novo Parkinson's Disease Patients With Depression. Frontiers in aging neuroscience. 2021;13:789785. 30. Hosokai Y, Nishio Y, Hirayama K, et al. Distinct patterns of regional cerebral glucose metabolism in Parkinson's disease with and without mild cognitive impairment. Movement disorders : official journal of the Movement Disorder Society. 2009;24(6):854-62. 31. Hirano S, Shinotoh H, Eidelberg D. Functional brain imaging of cognitive dysfunction in Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry. 2012;83(10):963-9. 32. Reeve A, Simcox E, Turnbull D. Ageing and Parkinson's disease: why is advancing age the biggest risk factor? Ageing research reviews. 2014;14:19-30.Figures
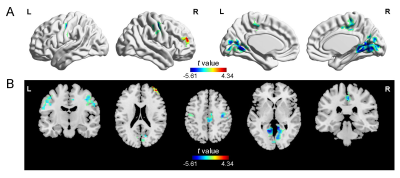
Figure 1. Voxel-wise intrinsic
timescales between the PD and HC groups. The PD group had shorter
timescales in bilateral postcentral and precentral gyri, bilateral lingual and
calcarine gyri, and the right middle cingulum gyrus, and longer timescales in
the right middle frontal gyrus than the HC group. HC, healthy controls; L, left; PD, Parkinson’s
disease; R,
right.
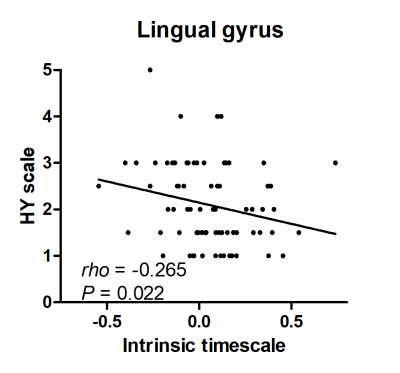
Figure 2. Correlation between the intrinsic
timescale and the symptom severity of PD. We found negative correlation between the intrinsic timescale of the lingual gyrus and
the scores of the Hoehn and Yahr (HY)
scale in
patients with Parkinson’s disease (PD).
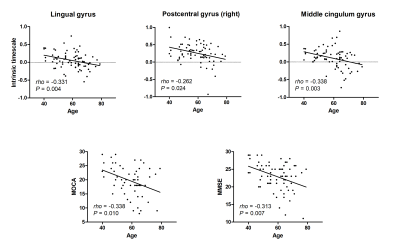
Figure 3.
Correlations between the intrinsic timescales and the age and between the
cognitive-related
scales and the age. We found negative correlation between the intrinsic timescales and the age in the lingual gyrus,
postcentral gyrus, and the middle cingulum gyrus for the PD group (Upper) and negative
correlation between the age and the scores of Montreal
cognitive assessment (MoCA) and Mini-mental state examination (MMSE) (Lower).
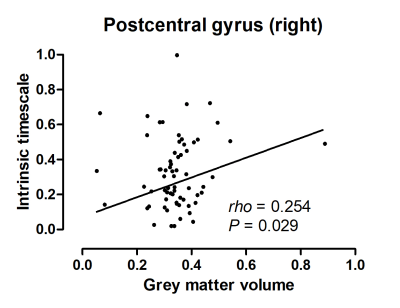
Figure 4. Correlations between the intrinsic
timescale and the grey matter volume. Shorter intrinsic timescale in
the right postcentral gyrus correlated with its reduced grey matter volume.
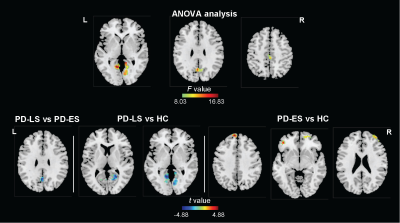
Figure 5. Voxel-wise intrinsic
timescales in PD at different stages. ES, early stage; HC, healthy controls; L, left; LS, late stage; PD, Parkinson’s disease; R, right.
DOI: https://doi.org/10.58530/2023/2283