2281
Multiparametric Characterization of Surface-Based Morphological Brain Networks in Middle- to Late-Stage Parkinson's Disease1Department of radiology,Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 2Department of CT & MRI, The First Affiliated Hospital, College of Medicine, Shihezi University, Shihezi, China
Synopsis
Keywords: Parkinson's Disease, Microstructure
As the structural basis of functional networks, the topological organization of morphological brain networks in the surface space has not been explored in more advanced stages of PD. We constructed individual morphological networks by estimating interregional similarity distribution in different cortical surface-based indices from structural MRI of PD patients and healthy controls (HCs). Graph theoretical analysis was performed to detect PD-related alterations. Compared with HCs, PD patients showed lower local efficiency and clustering coefficient for gyrification index-based morphological brain networks. These findings extend our understanding of the neurobiology of network dysfunction in middle- to late-stage PD.
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder, related to abnormal brain connectivity 1. The prevalence of PD has been on the rise, with predictions of an increase to about 10 million patients globally by the year of 20302. The whole brain morphological covariance network method based on structural magnetic resonance imaging (sMRI) has been applied to the study of PD3-5. Studying morphological networks at the individual level could improve understanding of the disruption and clinical relevance of cortical network in PD patients, and might provide a non-invasive disease-related biomarker. We aimed to provide a comprehensive mapping of morphological network in patients with middle-to-late-stage PD. Graph-theoretical analysis including network metrics, hub identification and interregional morphological connectivity were utilized to explore topological organizations of PD patients and health controls (HCs).Methods
Fifty middle- to late-stage PD patients and forty-six HCs were recruited. The 3D T1-weighted brain volume (3D T1BRAVO) were acquired using a 3.0 T scanner (Discovery MR 750, GE Medical Systems, Waukesha, WI) with the 32-channel head coil. The 3D T1BRAVO images underwent standard processes using CAT12 based on SPM12. Estimation of cortical thickness (CT) and reconstruction of central surface were performed based on a projection-based thickness method6, and other surface-based parameters of sulcal depth (SD), gyrification index(GI), and fractal dimension (FD) were calculated for each participant in native surface space. We constructed four large-scale morphological brain networks for each participant based on their vertex-wise surface maps of CT, FD, GI, and SD. The sparsity-based thresholding procedure was employed to convert them to a set of binary networks. The GRETNA toolbox was used to quantify the topological properties of the binary networks. The demographic differences between PD patients and HC were analyzed using SPSS 22.0. For each type of morphological brain networks, between-group differences in the AUC of global and nodal network metrics were compared using nonparametric permutation tests. Partial correlation analyses were then conducted to assess the relationships between network measures showing significant group differences and clinical variables in PD group.Results
All types of morphological brain networks followed a small-world organization in both groups (Figure 1). The alterations in quantitative values of global network parameters in patients was significant. Specifically, compared with the HCs, the PD patients exhibited lower local efficiency, λ, and Cp for GI-based morphological brain networks (Figure 2). For each type of morphological brain networks, the regions with the values of nodal centrality metric (nodal betweenness, nodal degree and nodal efficiency) falling within the top 10% are considered as hubs. The spatial distribution of hubs in PD and HC groups is shown in Figure 3. Compared to healthy controls, PD patients also have some unique hub regions. Relative to the HCs, PD patients exhibited increased nodal efficiency in right superior parietal gyrus only for the SD-based morphological brain networks. The other reported results did not survive when the multiple comparison correction was performed. Multiple linear regression analysis revealed that Hoehn and Yahr stage values were negatively correlated with the nodal betweenness of right banks of superior temporal sulcus (r = -0.338, p = 0.020) and the nodal efficiency of left rostral middle frontal gyrus (r = -0.358, p = 0.014) for the CT-based morphological brain networks. However, these correlations did not survive multiple comparisons correction.Discussion
This study employed four cortical indices to investigate significant alterations in the topological properties of individual surface-based morphological networks in middle-to-late-stage PD. Firstly, it is noteworthy that the morphological network-level alterations associated with PD seem to depend on the choices of morphological index that were used to calculate interregional morphological similarities and network measures. Specifically, PD-related alterations in global network properties and disrupted interregional connectivity appeared to emerge only in GI-based index, manifested by lower measures of clustering coefficients, local efficiency, as well as normalized characteristic path length for GI-based morphological brain networks. Abnormalities in nodal centralities involving frontal and parietal cortical regions were found in PD patients relative to HCs. Cortical atrophy, reduced neuronal activity and metabolism may be associated with disruption of nodal centrality, leading to dysfunction of the nigro-striato-cortical circuits of motor processing7, 8. Additionally, negative correlations between the nodal betweenness of right banks of superior temporal sulcus as well as the nodal efficiency of left rostral middle frontal gyrus and Hoehn and Yahr stage were observed in PD patients for CT-based morphological brain networks. Relatively enhanced dorsolateral prefrontal cortex including rostral middle frontal gyrus activation has been discussed as a compensation for impaired executive control in early de novo PD patients9. For PD patients in the middle- to late-stage, alterations to the neural network activity of the above nodes may play an important role in the disease's progression.Conclusion
In conclusion, the surface-based morphological brain networks at the individual level could facilitate to understand structural substrate of intersubject variability in behavior and function from a network perspective. These findings are of great significance to elucidate underlying pathophysiology mechanisms, predict progression and select personalized treatment targets.Acknowledgements
NoneReferences
1. Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008;79:368-376.
2. Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology 2019;18:459-480.
3. Chou KH, Lin WC, Lee PL, et al. Structural covariance networks of striatum subdivision in patients with Parkinson's disease. Hum Brain Mapp 2015;36:1567-1584.
4. Xu X, Guan X, Guo T, et al. Brain Atrophy and Reorganization of Structural Network in Parkinson's Disease With Hemiparkinsonism. Front Hum Neurosci 2018;12:117.
5. Zhou C, Gao T, Guo T, et al. Structural Covariance Network Disruption and Functional Compensation in Parkinson's Disease. Front Aging Neurosci 2020;12:199.
6. Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. NeuroImage 2013;65:336-348.
7. Filippi M, Sarasso E, Piramide N, et al. Progressive brain atrophy and clinical evolution in Parkinson's disease. Neuroimage Clin 2020;28:102374.
8. Ruppert MC, Greuel A, Tahmasian M, et al. Network degeneration in Parkinson's disease: multimodal imaging of nigro-striato-cortical dysfunction. Brain 2020;143:944-959.
9. Martin JA, Zimmermann N, Scheef L, et al. Disentangling motor planning and motor execution in unmedicated de novo Parkinson's disease patients: An fMRI study. NeuroImage: Clinical 2019;22.
Figures

Figure 1 Small-world parameters of whole-brain morphological networks play as a function of sparsity thresholds. All types of individual morphological brain networks exhibited higher clustering coefficient (γ > 1) and approximately equal characteristic path length (λ ≈ 1), indicating small-world organization. PD, Parkinson’s disease; HCs, health controls; γ, normalized clustering coefficient; λ, normalized characteristic path length.
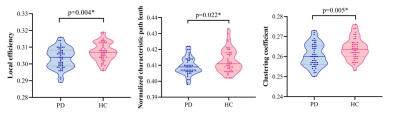
Figure 2 Differences in global topological properties of the GI-based morphological networks between PD and HCs. Compared with HCs, the PD patients exhibited significant decreases in local efficiency, normalized characteristic path and clustering coefficient. PD, Parkinson’s disease; HCs, healthy controls; GI, gyrification index.
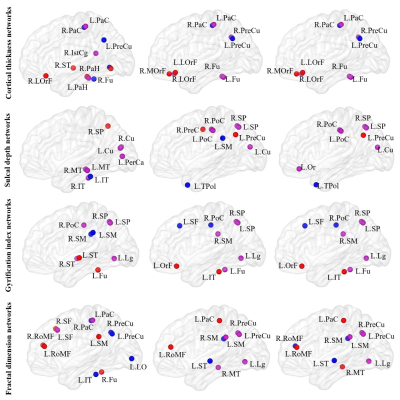
Figure 3 Hubs in each type of morphological brain networks for PD and HCs groups; The hub regions common to both groups is presented in purple. The hubs unique to the PD compared with HC is shown in red, and the hub specific to HC is shown in blue. LorF, lateral orbitofrontal; OrF, orbitofrontal; RoMF, rostral middle frontal; MOrF, medial orbitofrontal; PaH, parahippocampal; ST, superior temporal; MT, middle temporal; IT, inferior temporal; Fu, fusiform; and so on. L, left; R, right.