2277
Construction of disease progression prediction model for PD patients based on DTI data1Guangzhou First People's Hospital, Guangzhou, China, 2Guangzhou Women and Children's Medical Center, Guangzhou, China, 3The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China, 4Philips Healthcare, Guangzhou, China
Synopsis
Keywords: Parkinson's Disease, Machine Learning/Artificial Intelligence, white matter connectivity, DTI, prediction model
In this study, the machine learning method was used to establish a prediction model for Parkinson’s disease(PD) progression by using white matter connectivity and clinical information. A total of 123 PD patients were included. White matter network connection analysis and clinical information collection were performed for each patient. The results showed that combined with the white matter connection and clinical features, a good model of PD disease progression was established. White matter network connectivity helps predict PD progression at the individual level.Introduction
PD is a common progressive neurodegenerative disease, and its disease progression is a long and progressive process. Early prediction of PD is essential to implement appropriate interventions, however, reliable clinical outcomes and/or biomarkers of progression are still lacking[1]. Studies have shown that white matter microstructure changes occur before cortical neuron loss, and some of them have used DTI to observe the damage of white matter microstructure in PD patients[2]. Unlike previous studies, this research hypothesized that the clinical prognosis was predicted by a comprehensive assessment of the whole brain pattern, rather than analyzing the subtle changes of white matter from a global microscopic perspective through a little prior knowledge related to the etiological region[3]. Predictive and prognostic models are an important component in clinical practice, and high-precision and reliable models are needed to improve decision-making. Machine learning can greatly aid this process by automatically building a classification or prediction algorithm to capture robust statistical patterns in the data[4, 5]. Therefore, this study intends to adopt a longitudinal study design and use machine learning methods to analyze the whole-brain white matter network connectivity of PD patients, aiming to establish a prediction model based on whole-brain white matter connectivity and containing clinical information to predict the future clinical progress of PD patients.Methods
The data of PD patients in the PPMI(Parkinson's Progression Markers Initiative) database were evaluated. According to the Hoehn-Yahr scale (HYS) (stages 1-5) within 5 years, 49 progressed PD patients and 74 stable PD patients were enrolled in the current study. PANDA software was used to analyze the network connection of DTI data for each subject in the baseline. The Elastic Net-based feature consensus ranking (ENFCR) was used for feature ranking, and the prediction performance of four commonly used machine learning models (linear discriminant analysis, LDA; support vector machine, SVM; K-nearest neighbor, KNN; Naive Bayes, NB) was compared. (Fig.1) The single prediction model and combined model were constructed directly by combining the white matter network connectivity characteristics and clinical characteristics.Results
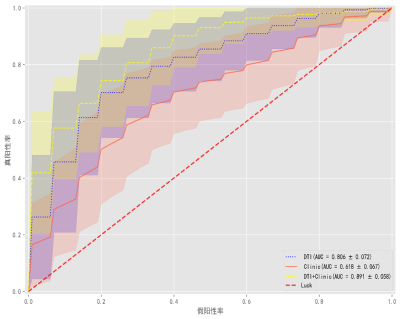
Among the four machine learning models, LDA had better prediction performance. The model using white matter network features alone (AUC=0.806±0.072) and the model using clinical features alone (AUC=0.618±0.067) showed moderate performance, while the model performance improved when they were combined (AUC=0.891±0.058).Discussion
Due to the high feature dimension of the whole brain white matter connectivity matrix, traditional statistical methods are very challenging and may lead to overfitting of the data. In this study, an enhanced elastic net feature ranking algorithm was used. Compared with other methods, this method can reduce the dimensionality of the sample dataset more effectively and improve the prediction accuracy of disease diagnosis, so as to realize the effective recognition of the key features of high-dimensional, small-sample, and unbalanced data. Our results showed that only the UPDRS III score was the only clinical feature beneficial to our model after feature screening. The MDS Unified Parkinson's Disease Rating Scale (MDS-UPDRS) is the most commonly used clinical scale to evaluate the severity of PD, which can comprehensively and in-depth evaluate the severity of PD. Our study suggests that it’s a biological indicator that can reflect the progression of PD. The combined model included eight parameters of white matter brain network connectivity. The FL and FN values of the thalamic subinterval have a high weight among these features of white matter brain network connectivity. Many studies have shown that structural abnormalities in the thalamus can also lead to these motor symptoms, and as the most important transit station in the brain, the thalamus may also be involved in the development of PD. In addition, the subinterval connectivity of the insula and its connection with external brain regions also play a certain role. Recent studies have shown that the insula plays an important role in processing subjective awareness and integration[6]. Degeneration of the insula dopaminergic, cholinergic, and serotonergic pathways in PD patients may have a significant impact on the functional integrity of the insula region[7]. Therefore, we can also speculate that subtle brain connectivity changes in subintervals of the thalamus and insula may occur prior to morphological changes and potentially predict disease progression in PD.Conclusion
White matter network connectivity contributes to the prediction of PD progression at the individual level, and when combined with clinical features, prediction performance was improved.Acknowledgements
No acknowledgement found.References
[1] Latourelle JC, Beste MT, Hadzi TC, et al. Large-scale identification of clinical and genetic predictors of motor progression in patients with newly diagnosed Parkinson's disease: a longitudinal cohort study and validation. Lancet Neurol. 2017. 16(11): 908-916.
[2] Tsai CC, Lin YC, Ng SH, et al. A Method for the Prediction of Clinical Outcome Using Diffusion Magnetic Resonance Imaging: Application on Parkinson's Disease. J Clin Med. 2020. 9(3).
[3] Park YW, Shin NY, Chung SJ, et al. Magnetic Resonance Imaging-Visible Perivascular Spaces in Basal Ganglia Predict Cognitive Decline in Parkinson's Disease. Mov Disord. 2019. 34(11): 1672-1679.
[4] Barrett MJ, Blair JC, Sperling SA, Smolkin ME, Druzgal TJ. Baseline symptoms and basal forebrain volume predict future psychosis in early Parkinson disease. Neurology. 2018. 90(18): e1618-e1626.
[5] Ma LZ, Zhang C, Wang H, et al. Serum Neurofilament Dynamics Predicts Cognitive Progression in de novo Parkinson's Disease. J Parkinsons Dis. 2021. 11(3): 1117-1127.
[6] Xiao B, Tan EK. Thalamic pathways mediating motor and non-motor symptoms in a Parkinson's disease model. Trends Neurosci. 2022 . 10(4): 1235-1244.
[7] Guan XJ, Guo T, Zhou C, et al. A multiple-tissue-specific magnetic resonance imaging model for diagnosing Parkinson's disease: a brain radiomics study. Neural Regen Res. 2022. 17(12): 2743-2749.
Figures