2276
Resting state functional connectivity alterations in motor networks of Parkinson’s disease in different frequency bands1Department of Magnetic Resonance, Lanzhou University Second Hospital, Lanzhou, China, 2Philips Healthcare, Xi'an, China, 3Gansu Province Clinical Research Center for Functional and Molecular Imaging, Lanzhou, China
Synopsis
Keywords: Parkinson's Disease, Parkinson's Disease, Motor symptoms, Neural networks
The functional alteration of the substantia nigra (SN), basal ganglia (BG), thalamus, and cortex are key hallmarks of Parkinson’s disease (PD) patients. Based on resting state functional magnetic resonance imaging, we investigated the functional connectivity (FC) of “cerebello-SN-BG-thalamo-motor cortical” network in the conventional and slow-4, slow-5 bands. PD patients demonstrated extensive FC decrease at conventional band, including inter-network connections of thalamo-cerebello-BG circuits, and connections between SN-putamen and postcentral gyrus-cerebellum. Slow-4 showed more thalamo-BG changes, while slow-5 specific FC was mainly the "cerebello-SN-BG-thalamo" circuits. The frequency specific alterations in motor related circuits may help in understanding the neuropathological mechanisms of PD.Introduction
Degeneration of nigrostriatal dopaminergic neurons, and subsequent disruption of basal ganglia (BG)–thalamo-cortical circuits, have been identified as the basis of motor symptoms of Parkinson’s disease (PD). However, the exact interaction mechanisms involving the cerebellum, substantia nigra (SN), BG, thalamus, and motor cortex in PD patients remain unclear. Hence, we selected those structures as regions of interest (ROIs) to explore the functional alterations of network and capture the frequency specific connectivity patterns.Methods
This study included 46 PD patients from Lanzhou University Second Hospital, and 41 age-, sex-, education-matched healthy controls (HCs). MRI data were acquired on a 3.0T scanner (Ingenia CX, Philips healthcare, the Netherlands) with a 16-channel head coil. Resting state functional MRI (rs-fMRI) images were acquired by EPI sequence (180 volumes; 36 contiguous slices; FOV: 192×192 mm2; thickness: 3 mm; matrix: 64×64; TR: 2000 ms; TE: 20 ms, FA: 90°). High-resolution iso-voxel 3D-T1 images were acquired as well. Rs-fMRI data were preprocessed using DPABI1. 28 PD patients and 34 HCs passed the quality control and head-motion evaluation (head displacement ≤2mm, maximum rotation ≤2°) (Table 1). Image data were separately filtered using the typical band (0.01–0.1 Hz), slow-5 band (0.01–0.027 Hz), and slow-4 band (0.027–0.073 Hz)2. The following ROIs were selected from bilateral hemisphere: cerebellum from AAL3 atlas (IV-V, VI, VIII lobules), substantia nigra (SN) from probabilistic atlas, basal ganglia (BG) (caudate nucleus, putamen, pallidum) and motor cortex (precentral and postcentral gyrus, paracentral lobule) from the Desikan-Killiany atlas, and thalamus (16 subregions in both hemisphere) from Brainnetome atlas. Fisher z transformed Pearson’s correlation coefficient (r) was calculated and used as the connection strength between ROIs. Therefore, a 36×36 symmetric and weighted motor related network was obtained for each subject. To detect the altered FC patterns, we used the Network Based Statistic (NBS) in Gretna toolbox3, with 1000 permutations. The Plink and Pcomponent were initially set as 0.05 and were further explored under 0.01. Spearman’s partial correlations, controlling for age, sex, education, and head motion were performed between aberrant FC and clinical variables (UPDRS-III and MoCA) to investigate the potential relationships (FDR correction).Results
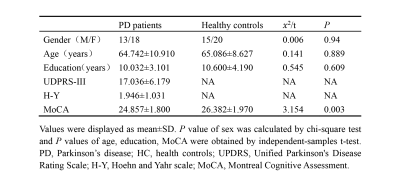
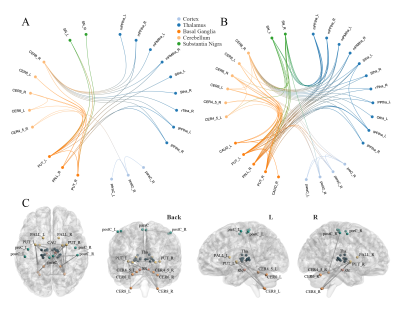
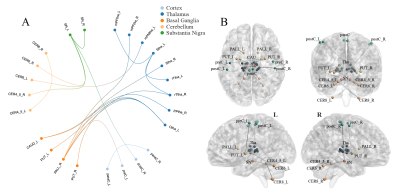
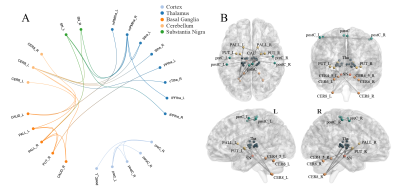
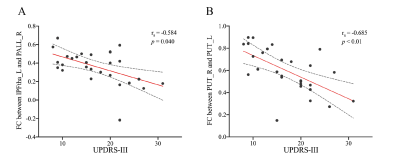
PD patients demonstrated extensively decreased FC in the conventional band. With the threshold of Plink and Pcomponent were both 0.05, PD patients showed one component (P=0.042) containing 28 ROIs and 74 edges, which involved the whole "cerebello-SN-BG-thalamo-motor cortical" network (Figure 1A). The component also reserved (P=0.017) under Plink=0.01 and Pcomponent=0.05, comprising 22 ROIs and 29 edges (Figure 1B), where the inter-network connections included: bilateral thalamus-right lobule VIII, bilateral thalamus-bilateral putamina and right pallidum, right lobule IV-V, bilateral lobule VI, VIII-bilateral putamina and right pallidum, and bilateral SN-putamen. While intra-network connections were the FC between bilateral lobules VI, between bilateral putamina, and between bilateral paracentral lobules and right postcentral gyrus. When comparing between groups in subfrequency band, with Plink and Pcomponent were both 0.05, we also found components of reduced FC (Pslow-4=0.036, Pslow-5=0.046). Compared with the component in the conventional band with the same P value, the slow-4 had more connections of thalamus-BG, along with decreased FC between right postcentral gyrus-left SN and left precentral gyrus-right putamen (Figure 2). While the slow-5 specific patterns were mainly the inter-network connections involving “cerebello-SN-BG-thalamo” network, and intra-network connections between motor cortices (Figure 3). Correlation analyses indicated negative relationships between UPDRS-III and both the FC of left lateral prefrontal thalamus-right pallidum (Spearman’s rho=-0.584, Pcorrected=0.040, Figure 4A), and the connection of bilateral putamina (Spearman’s rho=-0.685, Pcorrected<0.01, Figure 4B).Discussion
The dopamine neuron loss in the substantia nigra pars compacta is a pathological hallmark of PD. The striatal‐thalamo‐cortical (STC) circuits provides the path for BG to modulate cortical function, and the dysfunction of STC usually lead to bradykinesia and rigidity in PD patients4. While the cerebello‐thalamo‐cortical (CTC) circuitry highlights the role of cerebellum in tremor symptom of PD5. The decreased FC involving the SN-striatum, STC, and CTC circuits in our study may reflect the basis of the PD patient's motor and control impairment, which further validate the neural mechanism combining multiple circuits in PD.Low-frequency oscillations at different frequency bands exhibit different properties and physiological functions6. In the present study, the slow-4 and slow-5 specific FC patterns reflected the dysfunction of CTC and STC, respectively. Therefore, band separation may be sensitive in capturing the frequency dependent brain changes. Based on previous frequency related studies7, which focused on regional brain activity, our findings provide insights about altered functional coupling in different frequency bands.
With motor impairment worse, a decreased connectivity in the posterior putamen is the most consistent resting‐state functional alteration8, which was also detected in our study. Accordingly, the negative correlation between FC and motor scores may reflect a progressive loss of regulation in subcortical relay nuclei as the disease progresses, and underscore the potential value of these key structures as biomarkers for PD monitoring and prognostic assessment.
Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 81960309) and Gansu Province Clinical Research Center for Functional and Molecular Imaging (No. 21JR7RA438).References
1. Chao-Gan Y, Yu-Feng Z (2010) DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front Syst Neurosci 4:13. doi:10.3389/fnsys.2010.00013
2. Rong S, Zhang P, He C et al (2021) Abnormal Neural Activity in Different Frequency Bands in Parkinson's Disease With Mild Cognitive Impairment. Front Aging Neurosci 13:709998. doi:10.3389/fnagi.2021.709998
3. Wang J, Wang X, Xia M et al (2015) GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci 9:386. doi:10.3389/fnhum.2015.00386
4. Mcgregor M M, Nelson A B (2019) Circuit Mechanisms of Parkinson's Disease. Neuron 101:1042-1056. doi:10.1016/j.neuron.2019.03.004
5. Dirkx M F, Den Ouden H, Aarts E et al (2016) The Cerebral Network of Parkinson's Tremor: An Effective Connectivity fMRI Study. J Neurosci 36:5362-5372. doi:10.1523/jneurosci.3634-15.2016
6. Zuo X N, Di Martino A, Kelly C et al (2010) The oscillating brain: complex and reliable. Neuroimage 49:1432-1445. doi:10.1016/j.neuroimage.2009.09.037
7. Guan X, Guo T, Zeng Q et al (2019) Oscillation-specific nodal alterations in early to middle stages Parkinson's disease. Transl Neurodegener 8:36. doi:10.1186/s40035-019-0177-5
8. Herz D M, Eickhoff S B, Løkkegaard A et al (2014) Functional neuroimaging of motor control in Parkinson's disease: a meta-analysis. Hum Brain Mapp 35:3227-3237. doi:10.1002/hbm.22397
Figures