2274
Cerebral perfusion in Parkinson's disease with depression: an arterial spin-labeling magnetic resonance imaging study1Department of Radiology, Beijing Hospital, National Center of Gerontology, Beijing, China, 2Graduate School of Peking Union Medical College, Beijing, China, 3Siemens Healthcare GmhH, Erlangen, Germany, 4Siemens Healthineers International AG, Lausanne, Switzerland, 5MR Collaboration, Siemens Healthineers Ltd, Beijing, China
Synopsis
Keywords: Parkinson's Disease, Neurodegeneration, Depression
Arterial spin labeling-magnetic resonance imaging (ASL-MRI) combined with inline T1-weighted-based brain morphometry was used to evaluate regional cerebral blood flow in this study, which explored alteration of cerebral perfusion in Parkinson’s disease (PD) patients with depression and investigated its underlying neural mechanism. The results showed decreased cerebral perfusion in several brain regions in PD patients compared to healthy controls and a correlation between decreased cerebral perfusion of the right occipital white matter and right cingulate gyrus in PD patients with depression. This finding suggested that hypoperfusion of the limbic system is involved in the pathogenesis of PD with depression.Introduction
Depression, which increases patients' emotional burden, is common in Parkinson's disease (PD) and has an unclear neural mechanism1, 2. A large number of nuclear medicine studies available in depression reported blood flow abnormalities in a large array of brain regions3. Because of its noninvasive nature, MR-based perfusion technique such as arterial spin labeling (ASL) is increasingly being used to provide cerebral blow flow (CBF) quantification without the need for contrast administration. In this work, we hypothesized that cerebral perfusion alteration occurs in depression in PD patients and aimed to investigate the association of these alterations with the depression.Methods
A total of 34 PD patients [19 Parkinson's disease without depression (PD-n) and 15 Parkinson's disease with depression (PD-d)] and 30 gender- and age-matched normal controls (NC) were enrolled4. All participants signed an informed consent form before the examination. The depressive symptoms were defined according to the Hamilton Depression Rating Scale (HAMD). T1-weighted magnetization-prepared rapid acquisition gradient echo (T1w MP-RAGE) and pseudo-continuous ASL (pCASL) imaging were performed. All MR examinations were performed on a 3T MR scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a 64-channel head coil. The pCASL was performed using a research 3D turbo gradient spin echo (TGSE) sequence with the following parameters: TR/TE = 4350/20.9 ms, FOV=220 mm×220 mm, matrix=64×64 (interpolated to 128×128), bolus duration = 1800 ms, 16 label times (TI=800-3800 ms, interval time 200 ms), and acquisition time = 4 min 55s, including an M0 calibration volume. Regional CBF maps were automatically generated inline after data acquisition5. We set blood/tissue water partition coefficient lambda = 0.9 mL/g, labeling efficiency alpha = 60% (four background suppression pulses accounted for), T1-blood = 1650 ms, and T1-tissue = 1330 ms for perfusion quantification. The brain morphometry prototype MorphoBox was integrated into the T1-w MP-RAGE research application sequence with the following parameters: TR/TE = 2530/2.27 ms, TI=928ms, FA=8°, FOV =256mm×256mm, resolution = 1mm×1mm×1mm, and acquisition time = 5 min 12 s, providing a mask with the brain segmented into 48 regions, as shown in Figure 16. After registering the perfusion-weighted image to the T1-w image of each patient individually, the segmentation mask was used to extract regional mean CBF values. Previous studies have shown that decreased CBF in patients with depression occurs in specific brain regions. Therefore, we selected the bilateral cerebral hemispheres as regions of interest. All data were analyzed using SPSS (version 25.0) and MATLAB (MathWorks, Natick, MA, United States). An independent samples t-test was used to compare regional CBF values between the PD and NC groups, and the PD-n and PD-d groups. P-values were adjusted for false discovery rate in multiple comparisons. The associations between the HAMD score and CBF values of the brain were investigated using partial correlations. The level of significance was set at p < 0.05.Results
The demographics of the 64 participants are shown in Table 1. The differential CBF in different brain regions between individuals is shown in Figures 2 and 3. Compared with the NC group, the cerebral blood flow of the right putamen, left hippocampus, right insula, bilateral parietal lobes, occipital lobe, and temporal lobe gray and white matter was significantly lower in the PD group (P<0.05). Cerebral blood flow of the left thalamus left hippocampus, right cingulate gyrus, bilateral parietal gray matter, and bilateral occipital white matter were significantly lower in the PD-d group compared to the PD-n group (P<0.05). Additionally, the CBF values of the right occipital white matter (r=-0.370, P=0.034) and right cingulate gyrus (r=-0.410, P=0.018) were negative and correlated with the HAMD score (Table 2).Discussion
The ASL-MRI was used to evaluate the difference in CBF between the PD patients and NCs in the bilateral cerebral hemispheres in this study, showing that PDs had a predominant hypoperfusion pattern compared with the HCs. This finding could be due to the changes in motor patterns, cognitive function, and emotional state in PD patients7. The further study of the CBF changes in the PD-n and PD-d groups showed that the decreased cerebral perfusion of the limbic system-related brain regions was associated with depression. This concurs with previous findings suggesting that the limbic system might be an essential brain area involved in the pathogenesis of depression in PDs8, 9. However, we used a small size sample in this cross-sectional study; a longitudinal study with a larger sample size from multiple centers is warranted to further validate the current findings.Conclusion
This study demonstrated that hypoperfusion of PD patients in several brain regions might be one of the essential characteristics of this disease. In addition, there is a correlation between decreased cerebral perfusion of the limbic system and PD in patients with depression.Acknowledgements
No acknowledgement found.References
1. Tsai WC, Lin HC, Chang CC, et al. Neuropsychiatric symptoms in Parkinson's disease: association with caregiver distress and disease severity. Int Psychogeriatr Jun 2020;32(6):733-739.
2. Jones JD, Kurniadi NE, Kuhn TP, et al. Depressive symptoms precede cognitive impairment in de novo Parkinson's disease patients: Analysis of the PPMI cohort. Neuropsychology Nov 2019;33(8):1111-1120.
3. Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry 2000;48(8):813-829.
4. Starkstein S, Dragovic M, Jorge R, et al. Diagnostic criteria for depression in Parkinson's disease: a study of symptom patterns using latent class analysis. Mov Disord Oct 2011;26(12):2239-2245.
5. Buxton RB, Frank LR, Wong EC, et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med Sep 1998;40(3):383-396.
6. Schmitter D, Roche A, Maréchal B, et al. An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer's disease. Neuroimage Clin 2015;7:7-17.
7. Lin WC, Chen PC, Huang CC, et al. Autonomic Function Impairment and Brain Perfusion Deficit in Parkinson's Disease. Front Neurol 2017;8:246.
8. Imamura K, Okayasu N, Nagatsu T. The relationship between depression and regional cerebral blood flow in Parkinson's disease and the effect of selegiline treatment. Acta Neurol Scand Jul 2011;124(1):28-39.
9. Kim YD, Jeong HS, Song IU, et al. Brain perfusion alterations in depressed patients with Parkinson's disease. Ann Nucl Med Dec 2016;30(10):731-737.
Figures
Table 1. Demographics and clinical data under study. Data conforming to a normal distribution are expressed as x±s, and non-conformities are denoted by M (Q1, Q3). NC: normal control; PD: Parkinson' s disease;PD-n: Parkinson' s disease without depression; PD-d: Parkinson's disease with depression; ①: Chi-Squared tests; ②: Independent samples t-tests; ③: Mann-Whitney U test.
Table 2. Correlation analysis between the regional brain CBF value with significant perfusion differences and HAMD scale scores. *: There was statistically significant correlation with the HAMD scores through partial correlation analysis; HAMD: Hamilton Depression Rating Scale; LTc: CBF of left thalamus; LHc: CBF of left hippocampus; RPGc: CBF of right parietal gray matter; LOWc: CBF of left occipital white matter ROWc: CBF of right occipital white matter; RCc: CBF of right cingulate gyrus.
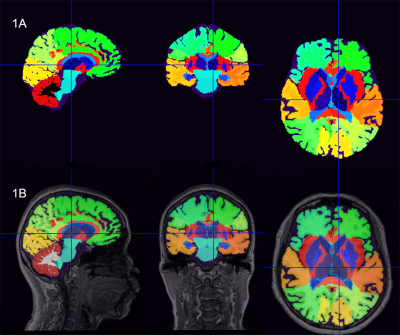
Figure 1. Schematic diagram of brain segmentation, with the images of a healthy subject's left thalamus as an example (female; 67 years old). (1A) Results of brain segmentation; (1B) Fused brain segmentation results onto 3D T1WI images.
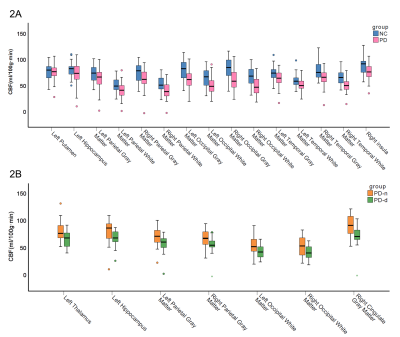
Figure 2. Boxplots of the CBF value in brain regions with significant perfusion differences. (2A) NC group vs. PD group; (2B) PD-n group vs. PD-d group.
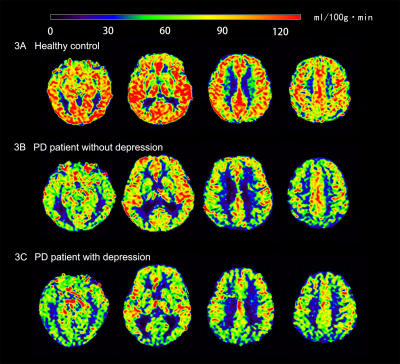
Figure 3. Representative axial slices of the brain cerebral blood flow images for (3A) a healthy subject (female; 67 years old), (3B) a typical PD-n group subject (male; 64 years old), (3C) a typical PD-d group subject (male; 75 years old). It is possible that (3B) and (3C) had decreased CBF in several brain regions compared to (3A); (3C) had decreased CBF in the occipital lobe and cingulate gyrus compared to (3B).