2270
Deep Learning Based on Knee MRI for Fully Automated Segmentation and Precision Diagnosis of Rheumatoid Arthritis, Gout and PVNS
Qizheng Wang1, Meiyi Yao2, Yandong Liu3, Xinhang Song2, Xiaoying Xing1, Yongye Chen1, Ke Liu1, Weili Zhao1, Xiaoguang Cheng3, Shuqiang Jiang2, and Ning Lang1
1Peking University Third Hospital, Beijing, China, 2Institute of Computing Technology, Chinese Academy of Sciences, Beijing, China, 3Department of Radiology, Beijing Jishuitan Hospital, Beijing, China
1Peking University Third Hospital, Beijing, China, 2Institute of Computing Technology, Chinese Academy of Sciences, Beijing, China, 3Department of Radiology, Beijing Jishuitan Hospital, Beijing, China
Synopsis
Keywords: Joints, Segmentation
Segmentation of synovial-related structures in MRI images can help assess synovitis-effusion, infrapatellar fat pad (IPFP) changes, and response to treatment, which is important for the clinical diagnosis of knee disease. However, segmenting images manually, which depends on the skill and experience of the physician; furthermore, it is time-consuming for radiologists. In this study, a deep learning pipeline for the 3D segmentation of the suprapatellar capsule (SC) and IPFP and knee synovitis classification were developed using proton density (PD)-weighted images of sagittal fat-suppressed knees, the most commonly used sequence in clinical practice, to support clinical decision-making.Purpose
Differential diagnosis of knee synovitis is important for early and effective treatment1-7. To develop a deep learning (DL) segmentation model of the suprapatellar capsule (SC) and infrapatellar fat pad (IPFP) based on knee MRI, and to establish classification models based on the two regions of interests (ROIs) to distinguish 3 common knee synovitis8-10. Their discrimination performance was compared with the radiologists' assessment.Methods
In this retrospective study, 376 patients (Internal training set: 233 cases, internal test set: 93 cases, external test set: 50 cases) with pathologically diagnosed knee synovitis, including rheumatoid arthritis (RA), gouty arthritis (GA) and pigmented villonodular synovitis (PVNS) from two institutions were included. Manual annotation was performed on SC and IPFP, and a semantic segmentation model was trained based on Resnet and UNet networks based on PD-weighted images to reduce the burden of manual annotation. The semantic segmentation network is followed by two pooling layers for feature extraction and further augmented by polynomial feature mapping with gender and age features for classification. The professional doctors' classification results were compared with five machine learning methods: support vector machine (SVM), multilayer perceptron (MLP), decision tree, AdaBoost, and XGBoost.Results
Results: Patients enrolled in institution A were assigned to a training cohort (69 with RA, 87 with GA, and 77 with PVNS) and a test cohort (25 with RA, 43 with GA, and 25 with PVNS). Patients included in Institution B formed the external test cohort (15 with RA, 21 with GA, and 14 with PVNS). The test results of the automatic segmentation model recall (internal test set=0.8164, external test set=0.7065) and mACC (I=0.9907, E=0.9893) all show a more accurate segmentation of ROI (IPFP and SC). Using the MLP classifier as an example, on the internal test set, the DL model showed better accuracy of 0.8566, Dice score of 0.7666, and AUC of 0.8264 than the accuracy of 0.7921, Dice score of 0.6904, and AUC of 0.7868 for the senior radiologist. On the external test set, the DL model was shown to have equal performance with the senior radiologist and better than the junior radiologist due to the accuracy of the classification (DL model = 0.7908, senior = 0.7867, junior = 0.7333), Dice score (DL= 0.6630, senior = 0.6684, junior = 0.6000) and AUC (DL= 0.7558, senior = 0.7674, junior = 0.7025).Conclusions
The established DL methods for segmentation and classification of different knee synovitis lesions based on SC and IPFP can support an accurate radiologic diagnosis.Acknowledgements
No acknowledgement found.References
- White C M, Kesler W W, Miner L, Flemming D. MR Imaging Knee Synovitis and Synovial Pathology. Magn Reson Imaging Clin N Am. 2022;30(2):277-291.
- Perry T A, Gait A, O'Neill T W, et al. Measurement of synovial tissue volume in knee osteoarthritis using a semiautomated MRI-based quantitative approach. Magn Reson Med. 2019;81(5):3056-3064.
- Wang Y, Teichtahl A J, Pelletier J P, et al. Knee effusion volume assessed by magnetic resonance imaging and progression of knee osteoarthritis: data from the Osteoarthritis Initiative. Rheumatology (Oxford). 2019;58(2):246-253.
- Perry T A, Yang X, van Santen J, Arden N K, Kluzek S. Quantitative and semi-quantitative assessment of synovitis on MRI and the relationship with symptoms in symptomatic knee osteoarthritis. Rheumatology (Oxford). 2021;60(4):1763-1773.
- Nieminen P, Hamalainen W, Savinainen J, et al. Metabolomics of Synovial Fluid and Infrapatellar Fat Pad in Patients with Osteoarthritis or Rheumatoid Arthritis. Inflammation. 2022;45(3):1101-1117.
- Mustonen A M, Kakela R, Lehenkari P, et al. Distinct fatty acid signatures in infrapatellar fat pad and synovial fluid of patients with osteoarthritis versus rheumatoid arthritis. Arthritis Res Ther. 2019;21(1):124.
- Roemer F W, Kassim Javaid M, Guermazi A, et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage. 2010;18(10):1269-1274.
- Mastboom M J L, Palmerini E, Verspoor F G M, et al. Surgical outcomes of patients with diffuse-type tenosynovial giant-cell tumours: an international, retrospective, cohort study. Lancet Oncol. 2019;20(6):877-886.
- VanItallie T B. Gout: epitome of painful arthritis. Metabolism. 2010;59 Suppl 1:S32-36.3.
- Zhou V Y, Lacaille D, Lu N, et al. Has the incidence of total joint arthroplasty in rheumatoid arthritis decreased in the era of biologics use? A population-based cohort study. Rheumatology (Oxford). 2022;61(5):1819-1830.
Figures
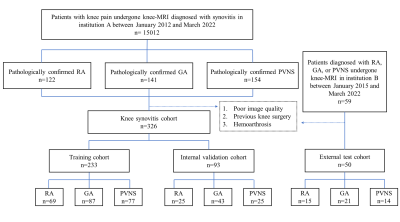
Figure 1. Flowchart of the study.
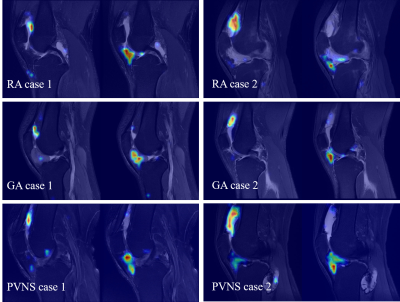
Figure 2. Grad-CAM of representative cases.
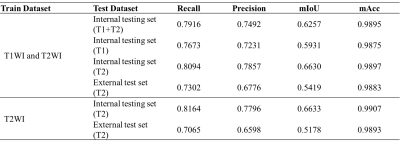
Table 1. Test results of automatic segmentation models.
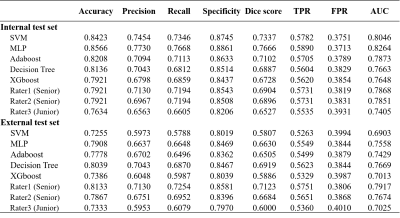
Table 2. Performance of five DL Model, radiologic resident, and musculoskeletal fellowship-trained radiologists for synovitis type classification on internal
external
test set.
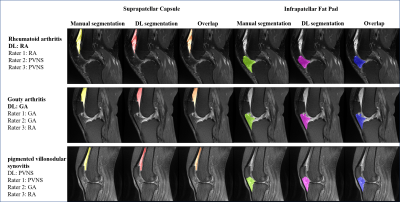
Figure 3: Representative cases of
segmentation and classification for knee synovitis in the test cohort.
Diagnosis by deep learning (DL) and 3 raters (Y.C. [rater 1], X.X. [rater 2], L.N. [rater 3])
are shown. RA = rheumatoid arthritis; GA = gouty
arthritis;
PVNS = pigmented villonodular synovitis.
DOI: https://doi.org/10.58530/2023/2270