2269
DCE-MR Lymphangiography Demonstrates Significantly Different Lymphatic Drainage in Patients with a FOXC2 Gene Mutation Compared to Controls1St George's University of London, London, United Kingdom, 2St George's University Hospitals NHS Foundation Trust, London, United Kingdom
Synopsis
Keywords: Vessels, Genetic Diseases, lymphatic
MRI can investigate lymphatic dysfunction and lymphoedema (a chronic swelling of interstitial fluid), however little quantitative research has been published. Dynamic Contrast-Enhanced Magnetic Resonance Lymphangiography (DCE-MRL) allows identification of lymph vessels in both healthy and lymphoedematous limbs, and was used to study the oedematous limbs of patients where the lymphoedema was associated with a mutation of the FOXC2 gene. Signal measured in leg lymphatics of these patients (n=6) peaked significantly earlier than in non-oedematous limbs (n=6) and demonstrated higher peak signals, despite an impaired lymphatic system and therefore an expectation of more sluggish drainage.Introduction
Lymphoedema, the chronic accumulation of interstitial fluid in bodily tissues, remains a poorly understood condition. Between 2013 and 2014, more than £178 million was spent on lymphoedema associated admissions in England, with similar sums spent on cellulitis admissions, a common co-morbidity of lymphoedema1,2. Studies investigating lymphatic function with MRI remain fairly rare however, 118 Magnetic Resonance Lymphangiography (MRL) studies are indexed on PubMed between 2019-2021, despite the potential the technique has shown in aiding lymphoedema diagnoses and assessing treatment response3,4. In this study employing dynamic contrast-enhanced MRL (DCE-MRL) in patients with Lymphoedema Distichiasis Syndrome (LDS), who have a confirmed mutation in the FOXC2 gene, we explore the temporal behavior of contrast uptake in lymphatic vessels in the lower leg to ankle region.Methods
An intradermal administration of a diluted gadolinium-based contrast agent (GBCA) solution (0.225M GBCA concentration) to each of the four interdigital spaces of the foot was performed, with local anaesthetic for pain reduction. DCE-MRL images from knee to ankle were obtained at 3.0T using a 3D T1-weighted sequence (spoiled gradient echo, TR/TE/FA = 3.7/1.6 ms/12o, SPAIR fat suppression, acquired voxel size = 1.0 mm isotropic, reconstructed = 0.7mm isotropic) for a minimum of approximately 27 minutes, and with a temporal resolution of ~ 3mins per volume5. Imaging was performed in patients with a confirmed FOXC2 gene mutation and six unaffected limbs; three from healthy controls and three from the unaffected limb of patients diagnosed with unilateral lower limb lymphoedema. Lymphatic vessels following the anteromedial drainage route6 were identified and signal measured within a lymphatic that was clearly resolved from other vascular structures. Adjacent muscle signal was measured at each timepoint and used to normalise lymphatic signal and account for any signal drift. ImageJ (version 1.53c) was employed for signal measurement. Elastic registration to the first post-contrast volume, using the freeware package NiftyReg7, was performed prior to all signal measurements5,8. Following signal measurement within 3 voxel3 regions, peak normalised lymphatic signal ($$$SI_{peak}$$$), time to peak signal ($$$T_{peak}$$$), and maximum enhancement ratio, $$MER=\max\left(\frac{SI_{k}}{SI_{1}}\right)$$ where $$$k$$$ signifies image number, were established. These values were compared via two-sample t-tests, following the establishment of normally distributed data, using SPSS (IBM, SPSS Statistics 27.0, Chicago, IL). $$$p < 0.05$$$ was considered significant.Results
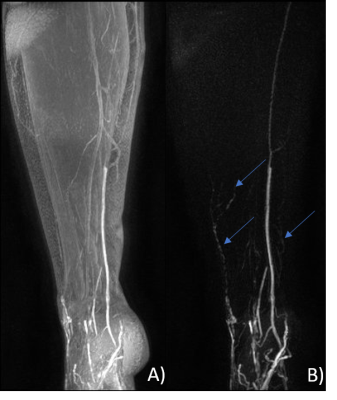
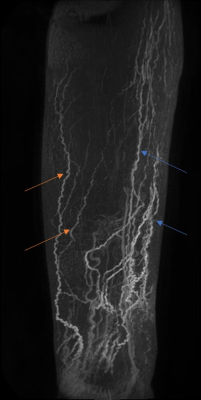
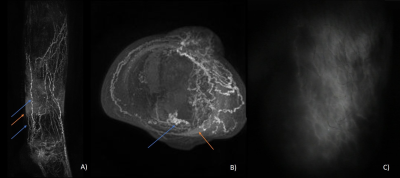
DCE-MRL was technically successful in all participants, with the slight discomfort of injection well tolerated and no adverse reactions reported. GBCA contrast-enhancement in lymphatic vessels following the anteromedial and anterolateral pathways were observed in both groups, as expected from interdigital injections, however many more vessels were seen in LDS patients (Figures 1-3). Surprisingly for interdigital injections, drainage via posterior drainage routes were evident in some LDS cases (Figures 3). These vessels often became evident after several minutes, with the anteromedial pathways typically visible from the commencement of imaging. Additionally, $$$SI_{peak}$$$, $$$T_{peak}$$$ and $$$MER$$$ were all significantly different at the $$$p < 0.05$$$ level. $$$T_{peak}$$$ was much reduced for patient limbs compared to controls (Figure 4), peaking on average within the first 10 minutes of imaging, with a corresponding reduction in $$$MER$$$ ($$$1.38 ± 0.41$$$ vs $$$1.91 ± 0.24$$$; unitless). $$$SI_{peak}$$$ was increased in LDS patients however ($$$3.31 ± 1.24$$$ vs $$$1.68 ± 0.72$$$; signal units).Discussion
FOXC2 gene mutations are associated with venous and lymphatic valve failures9, with retrograde lymphatic flow and dermal rerouting (lymphatic fluid entering the collecting lymphatics before flowing back toward the pre-collector vessels and then to the dermal lymphatic capillaries) having been demonstrated using Indocyanine Green Lymphography (ICG-L) in these patients (unpublished data). It is often considered self-evident that lymphatic flow rates will be reduced in cases of lymphoedema. However, our data suggests that in cases of LDS this may not be the case, given that signal in lymphatic vessels following the intradermal injection of a GBCA peaked significantly earlier than in non-oedematous limbs. That the signal is also higher in LDS suggests a more rapid transport of the GBCA via the anteromedial lymphatics, perhaps due to an increased interstitial pressure. To date we have not quantified signal changes in the lateral and posterior lymphatics, however visually these vessels appeared to enhance later than their medial counterparts. This behavior could suggest preferential initial drainage via the expected pathways (anteromedial), accompanied by a delayed filling of GBCA in the lateral and posterior vessels following retrograde flow and dermal rerouting from the collector vessels. Exploration of the applicability of existing DCE signal evolution models, accounting for the flux of contrast agent across lymphatic pools (e.g. dermal capillaries, deeper lying collector vessels etc.), should be explored in future studies, as should the effect of using the unaffected limb of unilateral lymphoedema cases as “control” limbs.Conclusion
DCE-MRL data suggests that lymphatic drainage in cases of LDS due to a FOXC2 gene mutation may be more rapid than in unaffected limbs when anteromedial vessels in the leg are interrogated. Given the poor temporal resolution of lymphoscintigraphy, and the inability to observe lymphatic vessels deeper than 1-2cm and behind areas of dermal rerouting in ICG-L (Figure 3), these results highlight the unique potential of MRI datasets in the quantitative assessment of lymphatic function in cases of lymphoedema.Acknowledgements
Research supported by the British Heart Foundation and Medical Research Council.References
1 - Citation: House of Lords debate, 9 September 2015, http://www.theyworkforyou.com/lords/?id=2015-09-09a.1457.3&3p=13471
2 - Atkin, Leanne. "Cellulitis of the lower limbs: incidence, diagnosis and management." Wounds UK 12.2 (2016): 52-56.
3 - Kim, Geunwon, et al. "MRI staging of upper extremity secondary lymphedema: correlation with clinical measurements." European radiology 30.8 (2020): 4686-4694.
4 - Forte, Antonio Jorge, et al. "Use of magnetic resonance imaging for evaluation of therapeutic response in breast cancer-related lymphedema: a systematic review." Archives of Plastic Surgery 47.04 (2020): 305-309.
5 - Mills, Michael, et al. "Image registration and subtraction in dynamic magnetic resonance lymphangiography (MRL) of the legs." BJR| case reports 8 (2022): 20210237.
6 - Shinaoka, Akira, et al. "Lower-limb lymphatic drainage pathways and lymph nodes: a CT lymphangiography cadaver study." Radiology 294.1 (2020): 223-229.
7 - Modat, Marc, et al. "Fast free-form deformation using graphics processing units." Computer methods and programs in biomedicine 98.3 (2010): 278-284.
8 - Mills, Michael et al. “Semi-Quantitative Analysis of Dynamic Contrast-Enhanced Magnetic Resonance Lymphangiography in the Legs of Primary Lymphoedema Patients”. Proceedings of the Annual Meeting of the ISMRM, London, 2022.
9 - Martin-Almedina, Silvia, Peter S. Mortimer, and Pia Ostergaard. "Development and physiological functions of the lymphatic system: insights from human genetic studies of primary lymphedema." Physiological Reviews 101.4 (2021): 1809-1871
Figures