2255
MR-Guided Cryoablation to Upregulate the Immune Response in Osteosarcoma
Dara L Kraitchman1,2,3, Michele Doucet4, Sarah J Powers3, Alainah Bhutta5, Emily Kulp6, Tina Ehtiati7, Cheri Rice1, Cindy Maranto1, Kathleen Gabrielson3, Cory Brayton3, and Brian Ladle4
1Russell H Morgan Department of Radiology and Radiological Science, Johns Hopkins University, Baltimore, MD, United States, 2Center for Image-Guided Animal Therapy, Baltimore, MA, United States, 3Department of Molecular & Comparative Pathobiology, Johns Hopkins University, Baltimore, MD, United States, 4Department of Oncology, Johns Hopkins University, Baltimore, MD, United States, 5University of Georgia College of Veterinary Medicine, Athens, GA, United States, 6Department of Chemical and Biomolecular Engineering, Johns Hopkins University, Baltimore, MD, United States, 7Siemens Medical Solutions USA, Inc., Baltimore, MD, United States
1Russell H Morgan Department of Radiology and Radiological Science, Johns Hopkins University, Baltimore, MD, United States, 2Center for Image-Guided Animal Therapy, Baltimore, MA, United States, 3Department of Molecular & Comparative Pathobiology, Johns Hopkins University, Baltimore, MD, United States, 4Department of Oncology, Johns Hopkins University, Baltimore, MD, United States, 5University of Georgia College of Veterinary Medicine, Athens, GA, United States, 6Department of Chemical and Biomolecular Engineering, Johns Hopkins University, Baltimore, MD, United States, 7Siemens Medical Solutions USA, Inc., Baltimore, MD, United States
Synopsis
Keywords: Bone, MR-Guided Interventions, cryoablation
Osteosarcoma (OSA) is the most common bone cancer in young adults and dogs and almost invariably lethal when the cancer spreads. MR-guided cryoablation offers the potential to cause direct bone necrosis and palliative pain management and has shown the potential to upregulate the immune response in prostate and breast cancer to prevent or shrink metastatic disease. The current study seeks to determine the immune response of MR-guided cryoablation in spontaneously occurring canine osteosarcoma in comparison to X-ray-guided intratumoral Stimulator of Interferon Gene (STING) immunotherapy as determine by survival time and measurement of inflammatory infilitrates.INTRODUCTION
Cryoablation is often used as a palliative treatment for bone metastases. Both lethal and non-lethal cooling cryoablation temperatures expose proteins that frequently illicit an immune response to shrink or prevent metastasis called the “abscopal effect.”1 Osteosarcoma (OSA) is the most common bone cancer in young adults and dogs and almost invariably lethal when the cancer spreads beyond the primary tumor site yet shows poor response to modern immunotherapies.2,3 The current study was designed to compare MR-guided cryoablation to intratumoral immunotherapy as a means to promote inflammatory infiltrates to stimulate the immune response in spontaneously occurring canine OSA.METHODS
All canine studies were approved by the institutional animal care and use committee. Dogs of either sex and any breed with radiographic and histopathology suggestive of appendicular OSA were recruited after obtaining Informed consent from all dog owners. Dogs were randomized to receive a single session of cryotherapy (Cryo) or two intratumoral injections of Stimulator of Interferon Genes (STING) agonist immunotherapy.An 18F-FDG PET-CT or whole-body CT was performed several days prior to treatment to confirm the absence of radiologically detectable metastatic disease. For MR-guided cryotherapy, a 1.5T proton density(PD)-weighted MRI scan (Siemens Espree) of the affected limb using a body matrix surface coil and spine array was performed of the anesthetized dog in the axial and sagittal planes for treatment planning with imaging parameters of: 5800 ms TR; 26 ms TE; 4 NSA; 18 ETL; 151 Hz/pixel BW; 4 mm slice thickness; 448x436 matrix; and 270 mm FOV. Typically, five contiguous axial images were then planned for the cryoablation needle path with the skin entry point marked by MR-visible fiducials. The body matrix was exchanged for a single loop coil, which was centered on the planned skin entry point. The skin was then sterilely prepared and draped, and a local anesthetic block was performed. Using an MR-conditional 4mm serrated drill, co-axial trocar sheath, and blunt ejector (In Vivo), the trocar-sheath was advanced into the osseous lesion using intermittent metal-artifact reducing, TSE MRI axial and sagittal images (1940 ms TR; 23 ms TE; 1 NSA; 24 ETL, 5 mm slice thickness, 280x280 FOV; 384x384 image matrix, and 407 Hz/pixel BW). Once the near cortex of the tumor was penetrated, the sheath was locked and the stylet was replaced with the bone drill to obtain biopsy specimens. After the biopsy specimens were obtained, an MR-compatible cryoablation needle (IceRod, Galil) was placed and at least two 10-minute freeze/5-minute thaw cycles were typically performed using an MR-compatible cryoablation system (Galil) while TSE images were acquired to document the extent of the iceball. The cryoablation needle and sheath were then removed and PD-MRI images in the axial and sagittal planes were repeated prior to recovery of the dog.
For the STING arm, a cone-beam CT (dynaCT body, Siemens Artis Zee) was performed to plan the skin entry point and trajectory of a standard bone biopsy device. The skin was sterilely prepared and draped followed by a local anesthetic block. The biopsy device was advanced under fluoroscopic guidance using the 3D overlay (i-pilot). Then 100 μl of ADU-S100 (Aduro) or an identical analog (InvivoGen) of STING agonist was injected intratumorally under X-ray guidance and repeated one week later. After treatment, all dogs received non-steroidal analgesics and oral antibiotics until amputation at ~2 weeks post-cryoablation. Histopathology was performed on the amputated limb to assess tumor type, degree of tumor necrosis, and inflammatory infiltrates. Follow-up imaging, i.e., CT or PET-CT, was performed at 3-, 6-, and 12-months post-amputation..
RESULTS
Six of seventeen OSA dogs were successfully treated with cryoablation with the remaining dogs receiving STING with same day discharge. Cryoablation was contraindicated in four dogs due to metal implants in the affected limb. Two dogs developed infections at the treatment site due to acral lick dermatitis. carboplatin chemotherapy (1-6 cycles) was performed in 13 dogs.Using MR-guidance, an oblique angulation with a single needle maximized treatment (Fig 1-4) and allowed 3D assessment of the extent of the iceball to prevent non-target freezing. However, due to the tumor size, complete ablation was not possible within 1-2 freeze-thaw cycles in any dog. Mean overall survival was increased in the STING treated dogs but not statistically significant due to the high variability in survival (Fig 5).
The ratio of T cells to myeloid cells is an important indicator of immune activation in tumors. STING treatment significantly increased the T cell to myeloid cell ratio in the tumors. Of particular note, the dog with greatest induction of T cell response had the longest survival time (1251 days with death not due to OSA.) However, necrosis from STING treatment was heterogeneous and localized only to the injection site in all dogs. Cryoablation showed large areas of necrosis, but in the dog with the longest survival time, no increase in T cell response was noted.
DISCUSSION AND CONCLUSIONS
MR-guided cryoablation of OSA was well tolerated but does not appear to have a direct upregulation of the immune response. Potentially combining cryoablation with STING agonist treatment may have a synergist effect to cause robust tumor necrosis with an augmented immune infiltrate upregulation.Acknowledgements
Grant support from Siemens, Boston Scientific Corporation/Galil, Swim Across America, The Children's Cancer Foundation, and NIH R01CA239124. The authors thank Inez Vazquez, Jennifer Lawyer, and Rebecca Krimins for assistance with the canine studies.References
1. Soanes WA, Ablin RJ, Gonder MJ: Remission of metastatic lesions following cryosurgery in prostatic cancer: Immunologic considerations. J Urol 1970 104:154-9.
2.Rowell JL, McCarthy DO and Alvarez CE. Dog models of naturally occurring cancer. Trends Mol Med. 2011;17:380-8.
3. Mirabello L, Troisi RJ and Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer. 2009;115:1531-43.
Figures
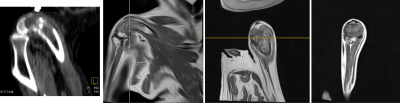
Fig1: Pre-treatment images of dog with osteosarcoma. CT image (left) provides limited ability to assess the extent of the bone tumor in comparison to Proton Density MRIs of distal femur ( L-->R: sagittal, coronal, and axial views)
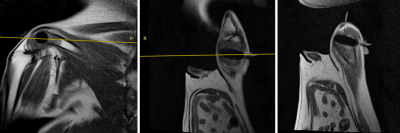
Fig 2: MRIs during first freeze. MRIs ( L-->R: Oblique sagittal, Oblique coronal, Oblique axial) acquired during 10-minute freeze showing iceball extent as a hyponintensity within the tumor in the same dog as Fig 1. Oblique imaging planes allow placement of the cryoablation probe to maximize the coverage of the tumor mass compared to CT-guided axial placement.
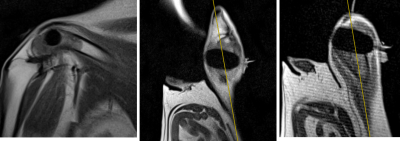
Fig 3: MRIs during second freeze. MRIs ( L-->R: Oblique sagittal, Oblique coronal, Oblique axial) acquired during second 10-minute freeze demonstrate further growth of the iceball. MR-guided monitoring prevents freezing of the skin margins at the edge of the needle.
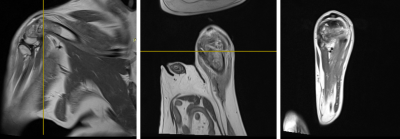
Fig 4: Post-treatment images of dog with osteosarcoma. Sagittal, coronal, and axial PD MRIs demonstrate that there are small artifacts associated with the bone drill/cryoablation needle, but no concern for hemorrhage or other adverse changes post-cryoablation in the same dog as Figs 1-3.
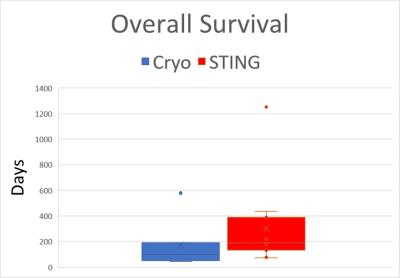
Fig 5: Box-whisker plot of overall survival. Overall survival post-amputation of dogs treated with cryoablation (blue) or STING agonist (red). While overall mean survival time in the STING agonist-treated dogs with osteosarcoma is increased relative to cryoablation (302 vs. 193 days), the wide variation in survival times suggests that there is a varied response to treatment.
DOI: https://doi.org/10.58530/2023/2255