2252
Tumor burden assessment in a SB CCA.Mdr2-/- cholangiocarcinoma mouse model with gadoxetate disodium-enhanced MRI at 9.4 T1Radiology, Beth Israel Deaconess Medical Center, Boston, MA, United States, 2Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA, United States, 3Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA, United States, 4Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Keywords: Liver, Tumor, preclinical models, cholangiocarcinoma
Cholangiocarcinoma (CCA) is a primary biliary malignancy with very poor prognosis. Early diagnostic detection and effective targeted therapies remain challenging. A novel hepatic fibrosis/CCA mouse model provides an investigative platform, but in vivo tumor detection/tracking is needed. Here we trial the use of in vivo MRI with gadoxetate disodium to detect CCA tumors and to differentiate infiltrative CCA from fibrosis in these models.Introduction
Cholangiocarcinoma (CCA) is the second most common primary hepatic tumor with increasing incidence worldwide.1,2 CCA is usually asymptomatic in the early stages and can be difficult to detect with conventional imaging, resulting in diagnosis commonly at an advanced, non-resectable stage. 3 The aggressiveness of the tumor, late diagnosis, and lack of effective treatment options result in an unfavorable prognosis, with 5-year survival rates of around 7-20%. 4 A new high-fidelity CCA mouse model, termed SB CCA.Mdr2-/-, recapitulates the increased susceptibility to CCA in the setting of progressive biliary injury and fibrosis observed in primary sclerosing cholangitis. There is currently a need to accurately and noninvasively track the progression or treatment response of these tumors, which can be either focal or infiltrative. 5 Gadoxetate disodium is a contrast agent that is selectively taken up by hepatocytes, resulting in increased T1 relaxation in normal liver relative to malignant tumors that do not take up the agent. The resulting T1 contrast between hepatocytes and non-hepatocytes is used clinically to detect primary or metastatic liver malignancies with MRI. 6,7 In this study, we performed liver gadoxetate disodium enhanced MRI on both fibrosis and CCA mouse models to see if MRI could detect discrete CCA lesions and to see if infiltrative CCA could be differentiated from fibrosis.Methods
Mouse model: Mdr2-/- mice spontaneously progress to severe biliary fibrosis. 8 Ten week old Mdr2-/- mice with congenital PSC-like progressive biliary disease were placed into two arms. Those Mdr2-/- mice without further treatment were placed in fibrosis group. CCA was induced in a second arm by subjecting Mdr2-/- mice to hydrodynamic tail vein injection of sleeping beauty transposon-transposase plasmid system with activated forms of AKT (myr-AKT) and Yap (YapS127A) protooncogenes (SB AKT/YAP1). Hydrodynamic tail vein injection of SB AKT/YAP1 plasmids has been shown to result in robust tumorigenesis in fibrotic Mdr2-/- mice, with 100% incidence and high CCA burden after 6 weeks.Image acquisition and analysis: Animals were imaged using a 9.4 T horizontal bore scanner equipped with a 36mm quadrature transmit/receive volume coil (Biospec 94/20, Bruker, Billerica MA). Animals were anesthetized with isoflurane in oxygen, given 0.05 mmol/kg gadoxetate disodium (Eovist, Bayer) by intraperitoneal injection and immediately situated at magnet isocenter with respiratory monitoring and thermal support to maintain body temperature 37 ℃. After acquisition of localizer images, a series of T1-weighted spoiled 3D gradient echo images was acquired with TR/TE=10/3 ms, 15º tip angle, and 120x100x32 matrix. Slice encoding was oriented head/foot, yielding 600µm axial slices with 250 µm in-plane resolution. Acquisition was respiratory gated with 60 dummy scans at the beginning of each acquisition window to maintain a consistent steady state. The duration of a single scan was approximately 4 minutes. T1-weighted imaging was initiated 5-10 minutes after contrast administration and continued until 40 minutes after the injection.
There were seven mice from each of the fibrosis and CCA arms. Images were reviewed by an abdominal radiologist with experience in both clinical and small-animal MRI, who was blinded to the study arm for each subject. To quantify liver enhancement, regions of interest (ROIs) were drawn over a homogenous region of the liver, excluding large vessels and bile ducts, and over muscle in the same image slice. To eliminate any effects of overall image scaling, the mean signal in the liver ROI was divided by the mean in the muscle ROI.
Results
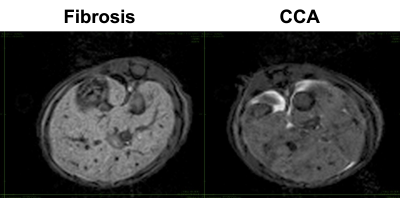
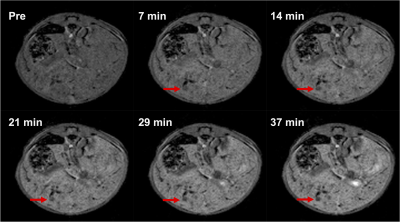
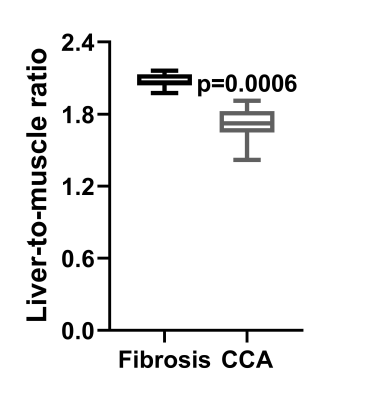
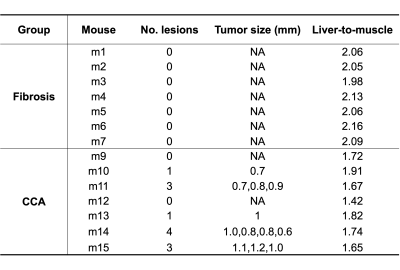
Figure 1 shows the steady-state phase (hepatocyte accumulation phase) following gadoxetate disodium administration in both fibrosis model and CCA model, approximately 40 minutes after injection. The higher signal intensity in the fibrosis animal relative to the CCA animal is apparent. Serial imaging of a mouse with CCA are shown in Figure 2, showing increased signal intensity of the hepatic parenchyma with gadoxetate disodium uptake and highlighting the presence of a hypointense focal CCA lesion (arrow). Five of 7 mice in the CCA group (71 %) demonstrated focal tumors measuring as small as 0.6 mm (Table 1). No lesion was detected in the fibrosis group. The liver-muscle signal ratio was significantly lower in CCA livers compared to fibrosis livers (2.073± 0.061 vs 1.706±0.155, n=7 per group, p=0.006 by non-parametric Mann-Whitney test, Figure 3).Discussion
MRI with gadoxetate disodium was able to detect focal CCA lesions. The overall decrease in hepatic signal intensity of the CCA livers as compared to fibrosis is suggestive of the detection of infiltrative CCA in this model. Intraperitoneal injection of gadoxetate disodium is relatively easier than intravenous administration, and long time-scale of hepatocyte uptake and retention (on the order of an hour or more) allows for a flexible imaging window. 9 Future studies will test the ability of MRI to track total tumor burden and response to investigative therapies.Conclusion
MRI with gadoxetate disodium can detect focal and infiltrative CCA in a SB CCA.Mdr2-/- mouse model, providing in vivo noninvasive tracking of tumor burden.Acknowledgements
No acknowledgement found.References
1. Raggi C, Taddei ML, Rae C, Braconi C, Marra F. Metabolic reprogramming in cholangiocarcinoma. Journal of hepatology. 2022;77(3):849-864. doi:10.1016/j.jhep.2022.04.038
2. Xu L, Wang L, Zhou L, et al. The SIRT2/cMYC Pathway Inhibits Peroxidation-Related Apoptosis In Cholangiocarcinoma Through Metabolic Reprogramming. Neoplasia (New York, NY). 2019;21(5):429-441. doi:10.1016/j.neo.2019.03.002
3. Guglielmi A, Ruzzenente A, Campagnaro T, et al. Intrahepatic Cholangiocarcinoma: Prognostic Factors After Surgical Resection. World journal of surgery. 2009;33(6):1247-1254. doi:10.1007/s00268-009-9970-0
4. Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. The Lancet (British edition). 2021;397(10272):428-444. doi:10.1016/S0140-6736(21)00153-7
5. Rahnemai-Azar AA, Weisbrod A, Dillhoff M, Schmidt C, Pawlik TM. Intrahepatic cholangiocarcinoma: Molecular markers for diagnosis and prognosis. Surgical oncology. 2017;26(2):125-137. doi:10.1016/j.suronc.2016.12.009
6. Mintorovitch J, Shamsi K. Eovist Injection and Resovist Injection: two new liver-specific contrast agents for MRI. Oncology (Williston Park, NY). 2000;14(6 Suppl 3):37-40.
7. Owen JW, Fowler KJ, Doyle MB, Saad NE, Linehan DC, Chapman WC. Colorectal liver metastases: disappearing lesions in the era of Eovist hepatobiliary magnetic resonance imaging. HPB (Oxford, England). 2016;18(3):296-303. doi:10.1016/j.hpb.2015.10.009
8. Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 ( Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. Journal of hepatology. 2005;43(6):1045-1054. doi:10.1016/j.jhep.2005.06.025
9. Leyendecker JRJR. Gadoxetate Disodium for Contrast Magnetic Resonance Imaging of the Liver. Gastroenterology & hepatology. 2009;5(10):698-698.
Figures