2249
Gd-EOB-DTPA Enhanced 3D T1WI: Utility of Compressed Sensing with Deep Learning Reconstruction for Liver Tumor Detection Improvement1Joint Research Laboratory of Advanced Medical Imaging, Fujita Health University School of Medicine, Toyoake, Japan, 2Radiology, Fujita Health University School of Medicine, Toyoake, Japan, 3Diagnostic Radiology, Hyogo Cancer Center, Akashi, Japan, 4Canon Medical Systems Corporation, Otawara, Japan, 5Fujita Health University Hospital, Toyoake, Japan
Synopsis
Keywords: Liver, Cancer
We hypothesize that CS with DLR can improve spatial resolution, image quality and tumor detection on Gd-EOB-enhanced 3D T1WI in suspected liver tumor patients. The purpose of this study was to determine the utility of CS and DLR for image quality and liver tumor detection improvements on high-resolution 3D CE-T1WI (HR-CE-T1WI) as compared with conventional 3D CE-T1WI with PI (conventional CE-T1WI) in patients with suspected liver tumors.Introduction
Contrast-enhanced (CE-) MRI with gadolinium ethoxybenzyl diethlenetriamine pentaacetic acid (Gd-EOB-DTPA) is currently one of the essential sequences and applied for liver tumor detection and evaluation in routine clinical practice. For Gd-EOB-DTPA enhanced T1-weighted imaging (T1WI) has been frequently obtained by means of 3D T1WI with conventional parallel imaging with or without breath holding. Since 2000s, conventional parallel imaging (PI) such as sensitivity encoding, etc. has been widely applied for improving temporal and spatial resolutions in routine MR imaging. In the last several years, compressed sensing (CS) with or without PI has been introduced by major MR vendors and applied in routine clinical practice (1-3). Moreover, deep learning reconstruction (DLR) has also been tested or applied to not only MRI, but also CT and suggested as useful for improving image quality for various clinical aims (4-7). However, CS has been mainly applied for improving temporal resolution for dynamic CE-MRI or reducing examination time in various organs. Moreover, DLR is applied for image noise reduction on MRI or CT, and little influence to diagnostic performance on MRI, except prostate DWI (5). Furthermore, no one has been assessed the utility of CS with DLR for high-resolution 3D CE-T1WI with Gd-EOB-DTPA to enhance liver tumor detection capability in patients with suspected liver tumors. We hypothesize that CS with DLR can improve spatial resolution, image quality and tumor detection on Gd-EOB-enhanced 3D T1WI in suspected liver tumor patients. The purpose of this study was to determine the utility of CS and DLR for image quality and liver tumor detection improvements on high-resolution 3D CE-T1WI (HR-CE-T1WI) as compared with conventional 3D CE-T1WI with PI (conventional CE-T1WI) in patients with suspected liver tumors.Materials and Methods
Seventy-seven patients (55 men, 22 women, mean age 63 years) with suspected liver tumor underwent dynamic CE-CT, 3D Gd-EOB-DTPA-enhanced T1WI at 3T MR systems (Vantage Centurian, Canon Medical Systems Corporation, Otawara, Japan) by conventional 3D CE-T1WI and HR-CE-T1WI with CS and reconstructed with DLR, surgical or interventional treatments, pathological examinations or more than 2 years follow-up examinations. In each patient, HR-CE-T1WI was obtained by static 3D T1-weighted fast gradient echo (FFE) sequence with CS and reconstructed with 1.5mm contiguous slice thickness by DLR method. On the other hand, conventional CE-T1WI was obtained by same sequence with conventional parallel imaging and reconstructed as 3mm contiguous slice thickness without DLR. The breath-holding time of each CE-T1WI was 28sec. Then, signal-to-noise ratio (SNR) of liver and contrast-to-noise ratio (CNR) between liver and tumor or cyst on each 3D CE-T1WI were assessed by ROI measurements. The probability of tumor on hepatobiliary phase was assessed on each CE-T1WI with 5-point scoring system by a board-certified abdominal radiologist with 28 years experiences and a board-certified chest radiologist with more than 28 years experiences of liver MRI. To determine the utility of CS with PI and DLR for improving image quality on each 3D CE-T1WI, SNRs and CNRs were compared between two methods by paired t-test. On comparison of tumor detection capability between two methods, Jackknife free-response receiver operating characteristic (JAFROC) analysis was performed to compare malignant tumor detection capabilities between two methods. Finally, figure of merit (FOM) values, sensitivity (SE) and false-positive rate/data set (FPR) for each radiologist and consensus assessment were also compared between two methods by using McNemar’s test or the signed rank test.Results
Representative cases are shown in Figures 1. Comparisons of SNR and CNRs between conventional CE-T1WI and HR-CE-T1WI are shown in Figure 2. SNR of HR-CE-T1WI was significantly higher than that of conventional CE-T1WI (p=0.002), although there were no significant differences of CNRs between tumor or cyst and liver (p>0.05). Comparisons of JAFROC analysis results and detection capability between conventional CE-T1WI and HR-CE-T1WI are shown in Figure 3. SEs or FPRs of HR-T1WI by consensus (SE: 0.90, FPR: 0.27/data set), reader 1 (SE: 0.92, FPR: 0.27/data set) and reader 2 (SE: 0.94) were significantly better than those of conventional CE-T1WI by consensus (SE: 0.84, p=0.004; FPR: 0.34, p=0.04), reader 1 (SE: 0.87, p=0.008; FPR: 0.34/data set, p=0.04) and reader 2 (SE: 0.89, p=0.02), although there were no significant difference of FOM between two methods at consensus (p=0.07), reader 1 (p=0.14) and reader 2 (p=0.10).Conclusion
Compressed sensing and deep learning reconstruction has a potential to improve image quality and liver tumor detection improvements on HR-CE-T1WI as compared with conventional 3D CE-T1WI in patients with suspected liver tumors.Acknowledgements
This study was technically and financially supported by Canon Medical Systems Corporation.References
- Feng L, Benkert T, Block KT, Sodickson DK, Otazo R, Chandarana H. Compressed sensing for body MRI. J Magn Reson Imaging. 2017;45(4):966-987.
- Ikeda H, Ohno Y, Murayama K, et al. Compressed sensing and parallel imaging accelerated T2 FSE sequence for head and neck MR imaging: Comparison of its utility in routine clinical practice. Eur J Radiol. 2021;135:109501.
- Choi MH, Kim B, Han D, Lee YJ. Compressed sensing for breath-hold high-resolution hepatobiliary phase imaging: image noise, artifact, biliary anatomy evaluation, and focal lesion detection in comparison with parallel imaging. Abdom Radiol (NY). 2022;47(1):133-142.
- Ueda T, Ohno Y, Yamamoto K, et al. Compressed sensing and deep learning reconstruction for women's pelvic MRI denoising: Utility for improving image quality and examination time in routine clinical practice. Eur J Radiol. 2021;134:109430.
- Ueda T, Ohno Y, Yamamoto K, et al. Deep Learning Reconstruction of Diffusion-weighted MRI Improves Image Quality for Prostatic Imaging. Radiology. 2022;303(2):373-381.
- Obama Y, Ohno Y, Yamamoto K, et al. MR imaging for shoulder diseases: Effect of compressed sensing and deep learning reconstruction on examination time and imaging quality compared with that of parallel imaging. Magn Reson Imaging. 2022;94:56-63.
- Matsuyama T, Ohno Y, Yamamoto K, et al. Comparison of utility of deep learning reconstruction on 3D MRCPs obtained with three different k-space data acquisitions in patients with IPMN. Eur Radiol. 2022;32(10):6658-6667.
Figures
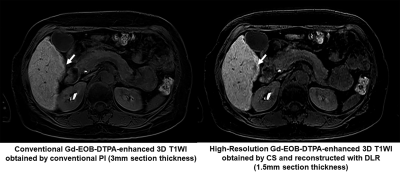
Figure 1. 67-year old male patients with pathologically proven hepatocellular carcinoma (HCC) in the S6.
Conventional CE-T1WI obtained with conventional parallel imaging, which is applied as standard protocol in routine clinical practice, can’t show any low intensity nodule in the S6. On the other hand, HR-CE-T1WI obtained by compressed sensing (CS) and reconstructed with deep learning reconstruction (DLR) clearly demonstrate small HCC (arrow) in the S6.
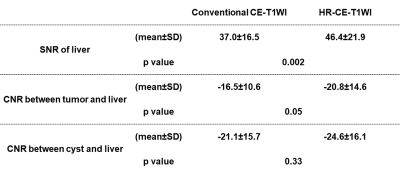
Figure 2. Comparisons of SNR and CNRs between conventional CE-T1WI and HR-CE-T1WI.
HR-CE-T1WI showed significantly higher SNR than conventional CE-T1WI (p=0.002), although no significant differences of CNRs between tumor or cyst and liver were observed (p>0.05).
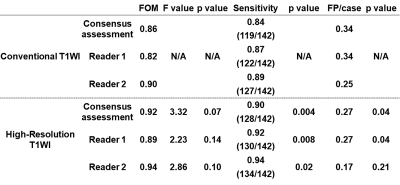
Figure 3. Comparisons of JAFROC analysis results and detection capability between conventional CE-T1WI and HR-CE-T1WI.
Sensitivities (SEs) or false-positive per rates (FPRs) of HR-T1WI by consensus, reader 1 and reader 2 were significantly better than those of conventional CE-T1WI by those evaluation (p<0.05).