2240
Multiparametric MRI manifestations of spontaneous intratumoral coagulative necrosis in hepatocellular carcinoma1Qilu Hospital of Shandong University, Jinan, China
Synopsis
Keywords: Liver, Cancer
Problem: To illustrate the MRI manifestations of spontaneous intratumoral coagulative necrosis (iCN) in hepatocellular carcinoma (HCC) and its value in predicting postoperative early recurrence. Methods: 263 patients with HCC confirmed by surgical pathology were enrolled. The qualitative assessments of MRI features were performed. Results: The iCN in HCC was characterized by a similar appearance to the tumor on pre-contrast MRI but with non-enhancement on post-contrast MRI. Presence of iCN on MRI was associated with early tumor recurrence. Conclusions: Presence of iCN on MRI is associated with early tumor recurrence and might assist to guide clinical treatment.Introduction
MRI technique may reflect tumor cellularity, which can be used to characterize benign and malignant liver lesions1. Substantial necrosis has been investigated in HCC on MRI2, 3, which is commonly defined as a central area of high signal intensity (SI) on fat-suppressed turbo spin-echo T2- weighted images (WI) without enhancement on postcontrast T1WI4. However, this manifestation is a typical feature of liquefactive necrosis on MRI. Whereas, intratumoral coagulative necrosis (iCN), another subtype of necrosis, is typically characterized by homogeneous clusters and sheets of dead and degraded tumor cells that coalesce into an amorphous coagulum5. The typical MRI characteristics of radiation-induced iCN in HCC6, 7 have been depicted as hyperintensity on T1WI and variable signal on T2WI without enhancement. However, the MRI features of spontaneous iCN in HCC is hardly reported in the literature. The purpose of our study was to investigate the MRI manifestations of spontaneous iCN in HCC and its value in predicting postoperative early recurrence.Methods
263 patients with HCC confirmed by surgical pathology (61 with iCN and 202 without iCN) who underwent preoperative multiparametric MRI between January 2015 and February 2019 were enrolled in this retrospective study. The inclusion criteria were as follows: (a) patients who underwent multiparametric liver MRI within 3 weeks before surgery, (b) patients without therapy (such as chemoembolization, radiation therapy and local ablation) before surgery, (c) patients with histology-identified spontaneous iCN and with MRI-defined substantial necrosis, and patients without necrosis ignored this item, and (d) patients with full histologic descriptions and adequate MRI imaging quality for analysis. The qualitative assessments of MRI features were identified including non-smooth tumor margin8, broken tumor capsule, nonrim arterial phase hyperintensity9, peritumoral arterial phase hyperenhancement9, and iCN. The signal intensity (SI) of iCN, surrounding tumor tissue, and neighboring normal liver and the ratios (iCN/tumor, iCN/liver, and tumor/liver) on T1- and T2- weighted images were measured and calculated, and so was the measurement of apparent diffusion coefficient (ADC) value. All patients underwent postoperative clinical and radiologic follow-up after surgical resection. Early recurrence (within the first 2 years) and patients with nonrecurrence or survival at the end of the follow-up period (the follow-up ended on March 31, 2020) were recorded. Kaplan-Meier method was used to compare patients with iCN or without iCN.Results
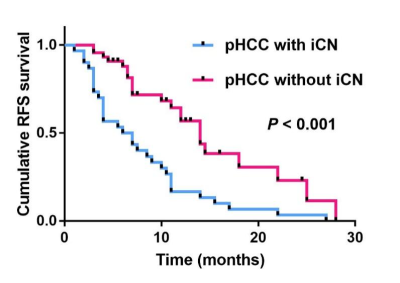
In assessments of MRI features related with early recurrence, tumor size >5cm (P<0.001), non-smooth tumor margin (P<0.001), broken tumor capsule (P=0.027), nonrim arterial-phase hyperintensity (P<0.001), and peritumoral arterial-phase hyperenhancement(P=0.013) were easily observed in HCC with iCN group than in HCC without iCN group. The iCN in HCC was characterized by similar appearances to tumor on pre-contrast MRI but with non-enhancement on post-contrast MRI (100%). No statistical differences in SIiCN and SItumor on T1WI and T2WI were significant, and so was the ADCiCN and ADCtumor (P>0.05). Presence of iCN on MRI was associated with early tumor recurrence (P <0.001) at Kaplan-Meier survival analysis.Discussion
he MRI appearances of spontaneous iCN of HCC can be variable, manifesting as hypo- to iso- intensity on T1WI and heterogeneous iso- to hyper- intensity on T2WI and DWI in our study. This is similar to the previously reported pure coagulative necrosis type of hepatic solitary necrotic nodules (SNN), which presented as hypo-intense on T1WI and mainly iso-intense on T2WI relative to the liver parenchyma10. However, ablation-induced coagulative necrosis manifestations as typical hyper-intensity on T1WI and hypo-intensity on DWI6, 7, which may be attributed to the different necrosis mechanisms and histological component. Above factors may have contributed to the different MRI presentations, but all the coagulative necrosis is typical with non-enhancement on post-contrast MRI. In this study, we further investigated the correlation between iCN and postoperative early recurrence. Kaplan-Meier survival analysis demonstrated that the presence of iCN was associated with tumor early recurrence. Wei T et al2 demonstrated that the degree of HCC tumor necrosis was associated with aggressive tumor characteristics and poorer RFS (p<0.05). This was similar to our study. Although they mainly focused on substantial necrosis, it was not surprising in iCN, which was a subgroup of substantial necrosis. Previous studies of renal cell carcinoma and glioma suggested that iCN was an independent predictor of poor tumor prognosis11, 12. The exact mechanism of postoperative early recurrence of HCC with iCN is still unknown. It was hypothesized that iCN might damage the tumor vasculature, and thus promotes tumor recurrence and systemic spread13. In addition, the hypoxia in the necrotic microenvironment may upregulate hypoxia-inducible factor 1-a expression, as well as induce angiogenesis and epithelial-mesenchymal transition to enhance early recurrence and metastasis14. Therefore, iCN, a previously overlooked type of necrosis, needs more attention in the future in clinical practice.Conclusions
The spontaneous iCN in HCC is indistinguishable with surrounding tumor tissue in pre-contrast MRI, but with typical features of non-enhancement on post-contrast MRI. Presence of iCN is an imaging marker for predicting postoperative early recurrence.Acknowledgements
None.References
1.Lee S, Kim SH, Hwang JA, et al. Pre-operative ADC predicts early recurrence of HCC after curative resection. Eur Radiol. 2019;29(2):1003-1012.
2.Wei T, Zhang XF, Bagante F, et al. Tumor Necrosis Impacts Prognosis of Patients Undergoing Curative-Intent Hepatocellular Carcinoma. Ann Surg Oncol. 2021;28(2):797-805.
3.Chen J, Xia C, Duan T, et al. Macrotrabecular-massive hepatocellular carcinoma: imaging identification and prediction based on gadoxetic acid-enhanced magnetic resonance imaging. Eur Radiol. 2021;31(10):7696-7704.
4.Mulé S, Galletto Pregliasco A, Tenenhaus A, et al. Multiphase Liver MRI for Identifying the Macrotrabecular-Massive Subtype of Hepatocellular Carcinoma. Radiology. 2020;295(3):562-571.
5.Nguyen HS, Milbach N, Hurrell SL, et al. Progressing Bevacizumab-Induced Diffusion Restriction Is Associated with Coagulative Necrosis Surrounded by Viable Tumor and Decreased Overall Survival in Patients with Recurrent Glioblastoma. AJNR Am J Neuroradiol. 2016;37(12):2201-2208.
6.Marasco G, Colecchia A, Colli A, et al. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J Hepatol. 2019;70(3):440-448.
7.Li S, Shi S, Li A, et al. Diffusion-Weighted Magnetic Resonance Imaging in Assessment of Primary Liver Cancer after HIFU Treatment. J Coll Physicians Surg Pak. 2019;29(4):305-308.
8.Wei Y, Pei W, Qin Y, et al. Preoperative MR imaging for predicting early recurrence of solitary hepatocellular carcinoma without microvascular invasion. Eur J Radiol. 2021;138:109663.
9.Chernyak V, Fowler KJ, Kamaya A, et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018;289(3):816-830.
10.Wang LX, Liu K, Lin GW, et al. Solitary Necrotic Nodules of the Liver: Histology and Diagnosis With CT and MRI. Hepat Mon. 2012;12(8):e6212
11.Sengupta S, Lohse CM, Leibovich BC, et al. Histologic coagulative tumor necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer. 2005;104(3):511-520.
12.Yee PP, Wei Y, Kim SY, et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun. 2020;11(1):5424. Published 2020 Oct 27.
13.Sabo E, Boltenko A, Sova Y, et al. Microscopic analysis and significance of vascular architectural complexity in renal cell carcinoma. Clin Cancer Res. 2001;7(3):533-537.
14.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605-622.
Figures
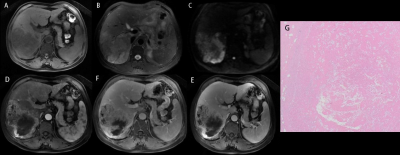
Figure1. A 63 aged man with a HCC in the right lobe. The MRI manifestations of iCN are hypointensity on T1WI (a), heterogeneous iso- to hyperintensity on T2WI (b), heterogeneous slight hypointensity on DWI (c) which are similar to surrounding tumor tissue, and nonenhancement on post-contrast MRI (d-f) but with relatively clear rim on delayed image (f). (g) was the iCN in pathology (HE, 10x10).