2238
Application of whole-tumor histogram analysis of restriction spectrum imaging in the diagnosis of benign and malignant liver lesions1Zhengzhou University People’s Hospital & Henan Provincial People’s Hospital, Henan, China, 2Henan University People’s Hospital & Henan Provincial People’s Hospital, Henan, China, 3Xinxiang Medical University & Henan Provincial People's Hospital, Henan, China, 4MR Collaboration, Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
Keywords: Liver, Cancer
Restricted spectrum imaging (RSI) is an advanced technique, which can detect the signal fractions of restricted, hindered and free diffusion of water molecules in the water compartments inside and outside the cell. However, the application of RSI in the assessment of liver disease is rare. Therefore, this study was to evaluate the diagnostic performance of RSI and DWI models in distinguishing benign and malignant liver lesions. Our results showed that the RSI model has better discriminative performance, and the combination of RSI parameters can further improve diagnostic performance. RSI provides a new perspective for exploring the information of tissue microenvironment, and its clinical application has broad prospects.Synopsis
Restricted spectrum imaging (RSI) is an advanced diffusion-based technique, which can detect the signal fractions of restricted, hindered and free diffusion of water molecules in the water compartments inside and outside the cell. However, the application of RSI in the assessment of liver disease is rare. Therefore, this study aimed to evaluate the diagnostic performance of whole-tumor histogram metrics derived from RSI compared with traditional DWI-derived metric in differentiating benign from malignant liver lesions. Our results showed that the RSI model has better discriminative performance than DWI, and the combination of RSI parameters can further improve diagnostic performance. RSI provides a new perspective for exploring the information of tissue microenvironment, and its clinical application has broad prospects.Introduction
Liver cancer is one of the most common and deadly malignant tumors in the world[1]. Due to the insidious onset of liver tumors and the lack of specific manifestations in the early stage, some patients often have advanced to the middle and late stages of diagnosis. The biopsy is the gold standard in diagnosis, but its high trauma and poor patient compliance limit its use as an early screening tool. Diffusion-weighted imaging (DWI) reflects the microstructure of biological tissues in vivo by quantitatively analyzing the diffusion motion of water molecules in tissues. The apparent diffusion coefficient (ADC) is the most commonly used quantitative parameter. DWI assumes that water molecules meet the Gaussian distribution in random motion. However, due to the complexity of human tissue structure, the diffusion motion of water molecules does not meet the Gaussian distribution[2]. To overcome this limitation, new diffusion models have been proposed, RSI model is one of them. The whole tumor histogram based on RSI provides a new perspective for exploring the information of tissue microenvironment. However, to the best of our knowledge, the use of the RSI model in liver tumors is rare. Therefore, the purpose of this study was to explore the value of a whole-tumor histogram based on the RSI model in differentiating benign and malignant liver lesions.Material and Methods
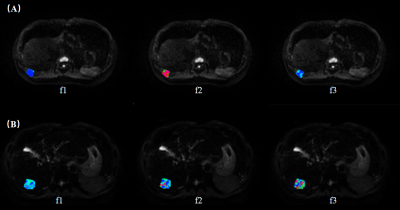
A total of 36 patients with suspected liver tumors who underwent abdominal MRI were included in this study. All the RSI images were acquired using a 3.0T MR scanner (uMR 790, United Imaging Healthcare, Shanghai, China) with 13 b values (b values: 0,25,50,100,150,200,400,600,800,1000,1500,2000,and 3000 s/mm2). Parametric maps were calculated by previously reported fitting methods (Figure 1). The apparent diffusion coefficient (ADC) and the restricted (f1), hindered (f2) and free diffusion (f3) parameters of the three-compartment RSI model were calculated. The intraclass correlation coefficient (ICC) was used to evaluate the consistency of the measured results. Independent sample t-test, Mann-Whitney U test, DeLong test and receiver operating characteristic (ROC) curve analysis were used for statistical evaluation.Results
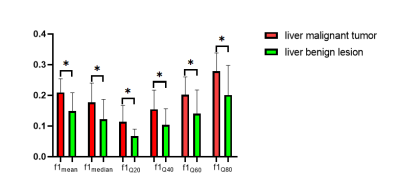
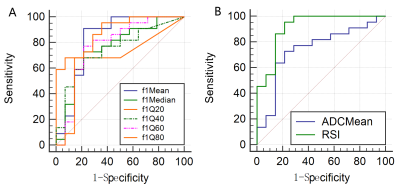
All parameters showed good agreement (ICC>0.75). The f1mean, f1median, f1Q20, f1Q40, f1Q60 and f1Q80 were higher and ADCmean was lower in the liver malignant tumor group than in the benign lesion group(P = 0.001, 0.010, 0.010, 0.013, 0.005, 0.002 and 0.014, respectively, Figure 2). The AUC of ADCmean is 0.744, the sensitivity is 72.73%, and the specificity is 78.57%. The combination of RSI parameters had the best discriminant performance, with an AUC of 0.916, the sensitivity of 95.45% and specificity of 78.57%. The ROC curves between the two groups showed that the combined parameters (f1mean+f1median+f1Q20+f1Q40+f1Q60)(area difference 0.172; P = 0.0066) was significantly higher than the AUC of ADC (Figure 3).Discussion
This study demonstrated the feasibility and validity of RSI model in differentiating benign from malignant liver lesions. f1 represents restricted diffusion of water molecules, reflects the cellularity of tumor[3,4]. The ADC is a quantitative parameter to assess the diffusion of water molecules in biological tissues, which can indirectly reflect the information of tissue structure and cell density at the micro-level[5]. In our study, the f1mean, f1median, f1Q20, f1Q40, f1Q60 and f1Q80 values were significantly higher and ADCmean was lower in malignant than benign liver lesions. This finding can be attributed to the rapid proliferation of malignant tumor tissues, with increased cell numbers, higher cell density, and limited diffusion distance of water molecules, and increased volume fraction of restricted diffusion in the microenvironment, resulting in higher f1 parameters and lower ADCmean in malignant liver lesions.Conclusion
The parameter f1 in the three-compartment RSI model can effectively distinguish benign and malignant liver lesions and is superior to the traditional ADC. The combination of multiple parameters can further improve the differential performance.Acknowledgements
No acknowledgementsReferences
1. Liu J, Li P, Wang L, et al. Cancer-Associated Fibroblasts Provide a Stromal Niche for Liver Cancer Organoids That Confers Trophic Effects and Therapy Resistance. Cell Mol Gastroenterol Hepatol. 2021;11(2):407-431.
2. Chaudhary N, Zhang G, Li S, et al. Monoexponential, biexponential and stretched exponential models of diffusion weighted magnetic resonance imaging in glioma in relation to histopathologic grade and Ki-67 labeling index using high B values. Am J Transl Res. 2021;13(11):12480-12494.
3. Xiong Z, Geng Z, Lian S, et al. Discriminating rectal cancer grades using restriction spectrum imaging. Abdom Radiol (NY). 2022;47(6):2014-22.
4. Brunsing RL, Schenker-Ahmed NM, White NS, et al. Restriction spectrum imaging: An evolving imaging biomarker in prostate MRI. J MAGN RESON IMAGING. 2017;45(2):323-36.
5. Usuda K, Ishikawa M, Iwai S, et al. Combination Assessment of Diffusion-Weighted Imaging and T2-Weighted Imaging Is Acceptable for the Differential Diagnosis of Lung Cancer from Benign Pulmonary Nodules and Masses. Cancers (Basel). 2021;13(7):1551.
Figures