2228
Reduction of artifacts and background signals in ex vivo mouse embryo MRI by potato starch suspension
Tomokazu Tsurugizawa1,2,3, Takuma Kumamoto4, and Yoshichika Yoshioka3,5
1Human Informatics and Interaction Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan, 2Faculty of Engineering, University of Tsukuba, Tsukuba, Japan, 3Center for Information and Neural Networks (CiNet), Osaka University and National Institute of Information and Communications Technology (NICT), Osaka, Japan, 4Developmental Neuroscience Project, Department of Brain & Neurosciences, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan, 5Graduate School of Frontier Biosciences, Osaka University, Osaka, Japan
1Human Informatics and Interaction Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan, 2Faculty of Engineering, University of Tsukuba, Tsukuba, Japan, 3Center for Information and Neural Networks (CiNet), Osaka University and National Institute of Information and Communications Technology (NICT), Osaka, Japan, 4Developmental Neuroscience Project, Department of Brain & Neurosciences, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan, 5Graduate School of Frontier Biosciences, Osaka University, Osaka, Japan
Synopsis
Keywords: Data Acquisition, Ex-Vivo Applications
The high-field MRI enables to investigate the microstructure in the mouse embryo. The proton-free fluid is used for the surrounding liquid around the specimen in MR-microimaging, but the potential issue of the image quality remains due to the air bubbles on the edge of the specimen and the motion artifact. Here, we demonstrated that the potato starch suspension with phosphate-buffered saline showed a low T1 and T2 signal intensity and strongly prevent the motion of the embryo during the scanning. These results indicate the utility of potato starch suspension for MR-microimaging of mouse embryos.Introduction
The microimaging of the mouse embryo, which is investigated to clarify the developmental mechanism of the brain, requires ex vivo ultrahigh-resolution imaging1. The proton-free fluids, such as Fluorinert and Fomblin, have been widely used in ex vivo MRI by filling around the tissue to reduce the susceptibility artifacts by the background signals. However, the potential issue of the image quality remains due to the air bubbles on the edge of the specimen2 and the motion. Additionally, if the water surrounding the tissue is not perfectly removed, the water proton residue remains around the tissue and disturbs the masking of the tissue. In the present study, we investigated the potato starch (PS) suspension with phosphate-buffered saline (PBS) for use as a surrounding liquid for ex vivo MRI by two features. We also investigated PS-PBS with gadolinium (Gd-PS-PBS) because Gd is used for ex vivo MRI.Methods
Animals and PS suspensionEmbryo (n = 2) C57BL/6J mice were sacrificed at embryo day 16 (E16). A whole body of E16 was fixed by 4% paraformaldehyde (PFA) and immersed in 5mM Gd solution (Gadodiamide Hydrate, 5mM) after fixation. PS suspension was made as the final ratio of PS and (Gd-)PBS (v/v) in PS suspension was 2:1.
MRI experiment
MRI acquisition was conducted at Bruker 11.7 T vertical magnet with a volume coil (inner diameter = 15 mm). A T1 of each material (PBS, Gd-PBS, PS power, PS-PBS, and Gd-PS-PBS) was measured using rapid acquisition with relaxation enhancement (RARE) with variable TR pulse (RARE-VTR) sequence with the following parameters: 8 TRs ranging from 40 ms to 5,000 ms, TE = 12 ms, spatial resolution = 195 × 195 µm²/pixel, slice thickness = 570 µm, and 1 slice. A T2 was measured using a multi-slice multi-echo (MSME) sequence with the following parameters: TR = 2500 ms, spatial resolution = 195 x 195 µm²/voxel, slice thickness = 570 µm, and 1 slice, with 16 TEs ranging from 10 to 160 ms for short TEs or 16 TEs ranging from 100 to 1,600 ms for long TEs. The high-resolution image of a Gd-penetrated embryo with Gd-PS-PBS was acquired using a three-dimensional fast low-angle shot (FLASH) with the following parameters: TE/TR: 6/40 ms, field of view = 15.0 × 15.0 × 9.0 mm3, spatial resolution = 29 × 29 × 50 µm3/voxel, flip angle = 90°, 8 averages (Total 8h11min31s). For the assessment of the body movement, the following parameters were used with 10 repetitions, TE/TR = 4/300 ms, spatial resolution = 78.1 × 78.1 µm2/pixel, slice thickness = 0.2 mm, 20 slices, 5 averages for FLASH and TE/TR = 46.9/5,000 ms, spatial resolution = 78.1 × 78.1 µm2/pixel, slice thickness = 0.2 mm, rare factor = 16, and 12 slices for RARE.
Image processing
Parametric T1 and T2 relaxation time was calculated from the averaged signal intensities within the region of interest, which was made by hand. The theoretical expression of T1 and signal intensity is, SI = M0 * (1 – exp [-TR/T1]), where SI is signal intensity, TR is repetition time, and M0 is maximum longitudinal signal intensity. The theoretical expression of T2 and signal intensity is SI = PD * exp (-TE/T2), where TE is echo time and PD is a proton density. The body movement of the embryo was investigated using the realignment processing program of SPM12. The six motion parameters, including the three translations and three rotations across the orthogonal axis, were calculated.
Results
The T1 and T2 relaxation were compared in PBS, Gd-PBS, PS powder, PS-PBS, and Gd-PS-PBS (Fig. 1). The T1-weighted signals of PS powder, PS-PBS, and Gd-PS-PBS were lower than those of PBS and Gd-PBS (Fig. 1A and Table 1). The T1 relaxation curves showed that the signal intensity of PS powder, PS-PBS, and Gd-PS-PBS was extremely lower than that of PBS and Gd-PBS (Fig. 1B). The T2 relaxation curve was also investigated using long TEs and short TEs in the same solutions (Fig. 1C, 1D, and Table 1), which showed that the signal intensity of PS powder, PS-PBS, and Gd-PS-PBS was higher than that of the background, but their signal intensity was extremely lower than that of PBS and Gd-PBS. The artifacts caused by the residual water, air bubble, and motion of the sample, were not perfectly excluded when using the non-proton liquid, but they were not observed when using PS-PBS or Gd-PS-PBS (Figs. 2 and 3). The motion of the tissue was suppressed during the scanning of RARE and FLASH with PS-PBS (Fig. 4). These results show the utility of PS suspension for ex vivo microimaging.Acknowledgements
No acknowledgement found.References
1. Petiet, A.E., Kaufman, M.H., Goddeeris, M.M., Brandenburg, J., Elmore, S.A., and Johnson, G.A. (2008). High-resolution magnetic resonance histology of the embryonic and neonatal mouse: a 4D atlas and morphologic database. Proc Natl Acad Sci U S A 105, 12331-12336. 10.1073/pnas.0805747105.
2. Scheffler, M., Maturana, E., Salomir, R., Haller, S., and Kovari, E. (2018). Air bubble artifact reduction in post-mortem whole-brain MRI: the influence of receiver bandwidth. Neuroradiology 60, 1089-1092. 10.1007/s00234-018-2071-8.
Figures
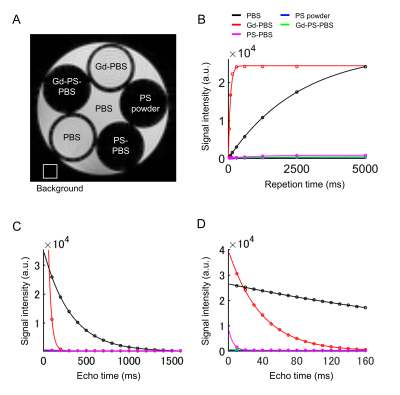
Figure 1 (A) T2-weighted image of the solution. (B) T1 relaxation, (C) T2 relaxation of each solution with long TEs, and (D) T2 relaxation of each solution with short TEs. The color lines indicate PBS (black), Gd-PBS (red), PS powder (blue), PS-PBS (magenta), and Gd-PS-PBS (green), respectively.
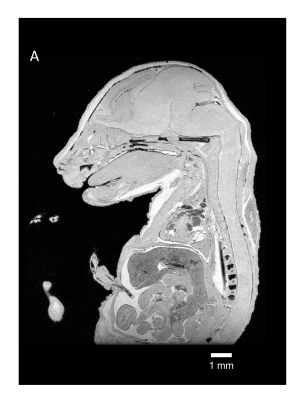
Figure 2 Ultrahigh-resolution image of mouse embryo with Gd-PS-PBS.
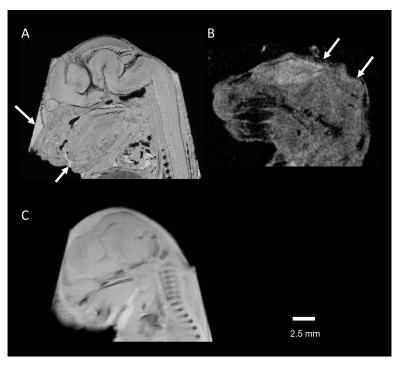
Figure 3 (A) Retained water on the surface of the tissue shows a high-signal intensity (arrow). (B) Susceptibility artifact caused by air bubbles on tissue-air interfaces at the tissue edges (arrow). (C) The blurred edge of the image is caused by tissue movements. These images were obtained using FC43 surrounding the embryo tissue.
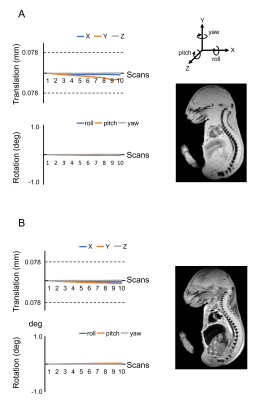
Figure 4 (A) Movement of the embryo body during 10 scans of T1-weighted imaging. Right figure shows the averaged T1-weighted image through 10 scans. (B) Movement of the embryo body during 10 scans of T2-weighted imaging. Right figure shows the averaged T2-weighted image through 10 scans.
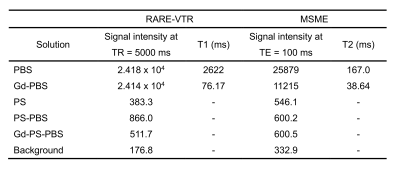
Table 1 signal intensity, T1 and T2 relaxation time
-: T1 relaxation times could not be calculated due to the low signal.
DOI: https://doi.org/10.58530/2023/2228