2218
Multi-Echo EPI performed on NexGen 7T scanner increases spatial resolution and shortens TE1Helen Wills Neuroscience Institute, University of California, Berkeley, CA, United States, 2Advanced MRI Technologies, Sebastopol, CA, United States, 3Radiology, University of California, San Francisco, CA, United States, 4San Francisco Veteran Affairs Health Care System, San Francisco, CA, United States, 5Siemens Medical Solutions USA, Inc., Malvern, PA, United States, 6Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Keywords: Data Acquisition, fMRI
Multi-echo (ME) EPI can increase BOLD sensitivity and reduce signal drop-out compared with standard gradient echo (GE) EPI, however spatial resolution and number of echo images are limited by the echo train length of each image. The powerful gradients on the NexGen 7T scanner allow for shorter echo spacing, and hence a greater number of echoes collected at high-resolution (1.6mm isotropic) as compared to standard 7T systems. ME-EPI collected at these resolutions can be separated into TE-dependent and TE-independent components using ME-ICA, showing promise for ME functional connectivity studies at 7T.Introduction
In standard 2D Gradient Echo (GE) EPI, an image is acquired at a single echo time (TE), chosen to maximize SNR and CNR in a given region. In Multi-echo (ME) EPI, multiple images are acquired at multiple TEs, which can then be combined to correct for susceptibility artifacts, improve BOLD sensitivity1,2, and to detect and remove non-T2* weighted noise components from the timeseries3,4,5. ME-EPI has been particularly powerful in functional connectivity studies6,7.ME-EPI segments the echo train into shorter echo trains, so time to encode spatial resolution per image is reduced, which constrains either the resolution or the number of echoes that can be acquired in a useful timeframe. This is a particular issue at 7T where the optimum TE is shorter than at 3T due to the reduced T2*. This echo train shortening can be mitigated by the use of parallel imaging techniques (e.g. GRAPPA), at the cost of reduced image SNR and increased g-factor noise. Thus the benefits of ME-EPI have been primarily shown at lower fields and/or resolutions (e.g ~2.5mm isotropic)8,9.
Here we investigate the capabilities of the NexGen 7T, with gradients designed for faster EPI readouts, to collect high-resolution, ME-EPI data.
Methods
MRI Scanner: Higher resolution is encoded without lengthening the echo spacing and echo train length using the high performance investigational “Impulse” head gradient (Siemens Healthcare, Erlangen, Germany)10 with slew rate 900 T/m/s and capable of a maximum gradient 200 mT/m. MRI data acquired using a 64 ch Rx, 8 ch Tx coil (MR CoilTec)11.Sequence: Data were collected using the Multi-Band (SMS) EPI 2D BOLD sequence, distributed via C2P from CMRR ported onto the MAGNETOM Terra Impulse edition NexGen 7T scanner (VE12U-AP02). Because of the high Gmax and Slew Rate achievable using the Impulse gradients, the echo spacing could be shortened and images were obtained at higher spatial resolutions and earlier TEs than had previously been achievable at 7T8,9.
EPI images were acquired at 1.6mm resolution using a range of different echo spacings, leading to a range of different TEs. Common parameters across scans: TR = 2s, SMS 3, GRAPPA 3, PF 6/8, 84 slices. Images were collected using a) bandwidths and echo spacings matched to those achievable on a standard Siemens Terra (0.59ms) b) moderate echo spacings achievable on the NexGen 7T (0.4 ms) and c) the shortest echo spacings achievable on the NexGen 7T (0.32 ms).
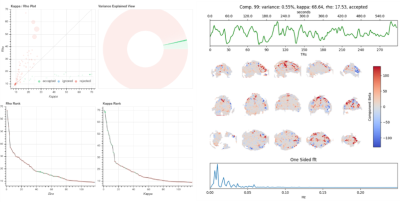
To assess image quality, tSNR was measured for each protocol both in the images collected at each TE, and in the images generated by the optimal combination of each timeseries using the T2* combination method2. Additionally, the protocol optimized for moderate echo spacings (0.4ms) was used to analyze both resting state and movie watching data using ME-ICA via the tedana toolbox12 to separate T2* weighted “BOLD” ICA components from non T2* weighted “noise” components3,4,5. A monoexponential model was fit to the data at each voxel using log-linear regression in order to estimate T2* and S0 maps. For each voxel, the value from the adaptive mask was used to determine which echoes would be used to estimate T2* and S0. ME data were then optimally combined2. PCA component estimation was applied to the optimally combined data for dimensionality reduction, and independent component analysis was then used to decompose the dimensionally reduced dataset. Kappa and Rho were calculated as measures of TE-dependence and TE-independence, and component selection was performed to identify BOLD (TE-dependent), non-BOLD (TE-independent), and uncertain (low-variance) components using the Kundu decision tree5.
Results and Discussion
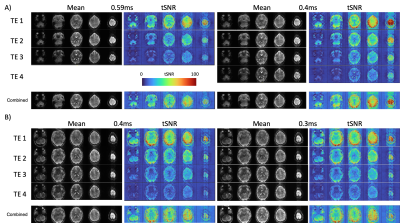
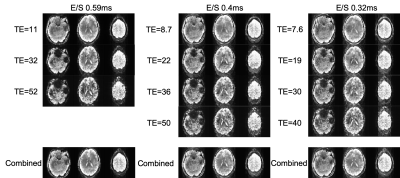
Figure 1 A) 0.4 vs 0.59ms echo spacing data shows increased tSNR and decreased signal dropout due to a shorter TE of the first echo image, and the ability to collect 4 echoes in the same time of recording 3 echoes using the longer echo spacing. Figure 1B) shows how the minimum TE and delta TE between echoes can be further reduced when echo spacing is reduced to the minimum of 0.32ms. At these echo spacings, subject dependent PNS becomes a major limiting factor, as does the increased ghosting arising from increased eddy currents when using such high gradient strengths (Figure 2). Additional sequence customization, such as the ability to independently control the amplitude and slew rate of specific parts of the EPI pulse sequence, and the use of dual polarity grappa techniques13, can mitigate these issues in the future.Even with these issues, ME-ICA can be used with this data to partition TE-dependent, BOLD-like components from TE-independent noise components in a resting state paradigms (Figure 3). Using the tedana pipeline12, ICA components from the ME-EPI dataset are assessed for Kappa (TE-dependence) and Rho (TE-independence), and accepted or rejected based on these measures. Accepted components for a resting state (Figure 3) task show expected features of brain networks after around 10 minutes of scanning.
Conclusion
The novel Impulse gradients on the NexGen 7T allow increased spatial resolution and additional echoes in ME-EPI compared to what is achievable on a standard 7T or 3T system. Further sequence optimization is possible to mitigate the remaining issues of ghosting and PNS at these very short echo spacings.Acknowledgements
This project is supported by the NIH BRAIN Initiative (R01MH111444, U01EB025162), 1R44MH129278. Samantha Ma is supported by Siemens Medical Solutions USA, Inc.References
1. Poser, B.A., Versluis, M.J., Hoogduin, J.M., Norris, D.G., 2006. BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: parallel acquired inhomogeneity desensitized fMRI. Magn. Reson. Med. 55, 1227–1235.
2. Posse, S., Wiese, S., Gembris, D., Mathiak, K., Kessler, C., Grosse‐Ruyken, M. L., ... & Kiselev, V. G. (1999). Enhancement of BOLD‐contrast sensitivity by single‐shot multi‐echo functional MR imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 42(1), 87-97.
3. Kundu, P., Inati, S.J., Evans, J.W., Luh, W.M., Bandettini, P.A., 2011. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. NeuroImage. 60, 1759–1770.
4. Kundu, P., Inati, S.J., Evans, J.W., Luh, W.-M., Bandettini, P.A., 2012. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. NeuroImage 60, 1759–1770.
5. Kundu, P., Brenowitz, N. D., Voon, V., Worbe, Y., Vértes, P. E., Inati, S. J., ... & Bullmore, E. T. (2013). Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proceedings of the National Academy of Sciences, 110(40), 16187-16192.
6. Lynch CJ, Power JD, Scult MA, Dubin M, Gunning FM, Liston C. Rapid Precision Functional Mapping of Individuals Using Multi-Echo fMRI. Cell Rep. 2020 Dec 22;33(12):108540.
7. Lynch CJ, Elbau I, Liston C. Improving precision functional mapping routines with multi-echo fMRI. Curr Opin Behav Sci. 2021 Aug;40:113-119.
8. Prantik Kundu, Valerie Voon, Priti Balchandani, Michael V. Lombardo, Benedikt A. Poser, Peter A. Bandettini, Multi-echo fMRI: A review of applications in fMRI denoising and analysis of BOLD signals, NeuroImage, Volume 154, 2017, Pages 59-80.
9. Morris LS, Kundu P, Costi S, Collins A, Schneider M, Verma G, Balchandani P, Murrough JW. Ultra-high field MRI reveals mood-related circuit disturbances in depression: a comparison between 3-Tesla and 7-Tesla. Transl Psychiatry. 2019 Feb 15;9(1):94.
10. Feinberg DA., et al., Design and Development of a Next-Generation 7T human brain scanner with high-performance gradient coil and dense RF arrays. ISMRM 2021
11. Gunamony S, and Feinberg DA "An 8-channel transmit 64-channel receive compact head coil for Next Gen 7T scanner with head gradient insert." ISMRM 2022
12. DuPre, E. M., Salo, T., Ahmed, Z., Bandettini, P. A., Bottenhorn, K. L., Caballero-Gaudes, C., Dowdle, L. T., Gonzalez-Castillo, J., Heunis, S., Kundu, P., Laird, A. R., Markello, R., Markiewicz, C. J., Moia, S., Staden, I., Teves, J. B., Uruñuela, E., Vaziri-Pashkam, M., Whitaker, K., & Handwerker, D. A. (2021). TE-dependent analysis of multi-echo fMRI with tedana. Journal of Open Source Software, 6(66), 3669.
13. Hoge WS, and Polimen JRi. “Dual-Polarity GRAPPA for Simultaneous Reconstruction and Ghost Correction of Echo Planar Imaging Data.” Magnetic Resonance in Medicine 76, no. 1 (2016): 32–44
Figures