2206
Fast, High-Resolution, and Optimal Contrast MRI
Yao Sui1,2, Onur Afacan1,2, Camilo Jaimes1,2, Ali Gholipour1,2, and Simon K Warfield1,2
1Harvard Medical School, Boston, MA, United States, 2Boston Children's Hospital, Boston, MA, United States
1Harvard Medical School, Boston, MA, United States, 2Boston Children's Hospital, Boston, MA, United States
Synopsis
Keywords: Image Reconstruction, Quantitative Imaging
Any MRI practices prefer high resolution, high signal-to-noise ratio, short scan time, and high contrast. Unfortunately, fast scan leads to low resolution while high-resolution scan results in a reduced signal-to-noise ratio. In particular, it is challenging for radiologists and technologists, who perform MRI scans, to find optimal sequence parameters for each patient, leading to sub-optimal contrast. We developed a new methodology that enables fast and high-resolution brain MRI with improved signal-to-noise ratio and optimal contrast between white-matter and gray-matter for each individual patient, based on quantitative imaging and reconstruction techniques. Experiments on clinical data demonstrated the advantages of our approach.Introduction
The trade-off between scan time, resolution, and signal-to-noise ratio (SNR) has been an inevitable matter in any MRI practices1. High-resolution imaging allows for delineations of fine anatomical structures but suffers from reduced SNR and requires increased scan time. Long scan time discomforts patients and potentially causes motion artifacts2. Meanwhile, it is challenging for radiologists and technologists, who perform MRI scans, to find optimal sequence parameters for each patient, leading to sub-optimal contrast. Super-resolution reconstruction techniques have emerged to offer high-resolution MRI with improved SNR and reduced scan time3,4. Quantitative imaging enables the connection from signal intensity to imaging model5, where proton density and T1/T2 mappings, which essentially infect the voxel values of an MRI image, can be estimated6,7. Therefore, an MRI image can be reconstructed from its T1/T2 mapping by adjusting the sequence parameters that have been set before the scan8,9. We developed a new methodology that enables fast and high-resolution brain MRI with improved SNR and optimal contrast between white-matter and gray-matter for each individual patient, based on quantitative imaging and reconstruction techniques. Our approach allows for performing MRI scans with sub-optimal parameter settings, and adjusting the parameters in the reconstruction for the optimal contrast tailed to each individual patient. Experiments on clinical data demonstrated the advantages of our approach.Methods
We acquire MRI images with variable inversion times (TI) and orientations. These images are of in-plane high-resolution (0.8mm x 0.8mm) and through-plane low-resolution (2 mm), so as to be scanned fast. We acquired a dataset from a clinical 3T MRI scanner that contains three sets of PSIR images with a flip angle of 158 degrees and TIs of 400, 800, and 1200 ms respectively. Each set includes an axial, coronal, and sagittal image.We perform a super-resolution approach3 to obtain a high-quality image at the isotropic resolution of 0.8 mm from each set of images. We then estimate the proton density ($$$pd$$$) and T1 ($$$T1$$$) mappings from the three super-resolved images, according to the T1 relaxation model$$s\left(TI,\alpha\right)=pd \cdot\left(1-\left(1-\cos\alpha\right)\cdot e^{-\frac{TI}{T1}}\right)$$There are two parameters we can adjust for voxel intensity $$$s$$$ in the model: the flip angle $$$\alpha$$$ and $$$TI$$$. We define the contrast between gray-matter and white-matter by$$contrast=\frac{\left|m_{GM}-m_{WM}\right|}{\sqrt{\sum_{i\in GM}\left(s_i\left(TI,\alpha\right)-m_{GM}\right)^2}+\sqrt{\sum_{i\in WM}\left(s_i\left(TI,\alpha\right)-m_{WM}\right)^2}}$$where $$$m_{GM}$$$ and $$$m_{WM}$$$ denote the means of voxel intensities from gray-matter and white-matter respectively, and $$$s_i(TI,\alpha)$$$ denotes the $$$i$$$-th voxel intensity. We perform a segmentation approach to obtain the two voxel index sets of gray-matter ($$$GM$$$) and white-matter ($$$WM$$$). We maximize the contrast between the gray-matter and white-matter with respect to both $$$TI$$$ and $$$\alpha$$$, using a Powell optimizer10. Consequently, we achieve the optimal contrast from $$$s(TI^{*}, \alpha^{*})$$$ with $$$TI^{*}$$$ and $$$\alpha^{*}$$$ maximize the contrast measure.Results
Figure 1 shows the low-resolution PSIR images from our clinical dataset. The in-plane resolution is 0.8mm x 0.8mm and the through-plane resolution is 2mm, acquired in axial, coronal, and sagittal planes with a flip angle of 158 degrees and inversions times of 400, 800, and 1200 ms. Figure 2 shows the super-resolved PSIR images at the isotropic resolution of 0.8mm with all three inversion times. The results suggest that our super-resolution approach offered high-quality images with improved SNR and precise anatomical structures. Figure 3 shows the estimated proton density and T1 mappings from the super-resolved images as well as the data fitting over the representative voxels from white-matter, gray-matter, and CSF, respectively, according to the T1 relaxation model. These results suggest that our approach generated accurate and high-resolution proton density and T1 mappings from the super-resolved images. Figure 4 shows the variations of contrast measures over flip angle and inversion time, which were computed from the estimated proton density and T1 mappings. The results suggest that the contrast measure is eligible to be maximized over inversion time and flip angle. Figure 5 shows the reconstructed PSIR image with optimal contrast between gray-matter and white-matter. The optimal contrast was achieved at the inversion time of 312 ms and flip angle of 142 degrees. Interestingly, when we constrained TI to be large (>2500 ms), the reconstructed PSIR image exhibited a T2-like contrast with an inversion time of 23428 ms and flip angle of 3.8 degrees for optimal contrast. Note that it is not reasonable to have such a large TI (23428 ms) in practice. This just shows the advantage of our computational approach to reconstruct the image with arbitrary large TI values. The results show that our approach offered high-resolution, high SNR, and optimal contrast PSIR images, while in parallel, reducing the scan time.Discussion
We have developed a new methodology that enables fast and high-resolution brain MRI with improved signal-to-noise ratio and optimal contrast between white-matter and gray-matter for each individual patient, based on quantitative imaging and reconstruction techniques. We have demonstrated that our approach allows for performing MRI scans with sub-optimal parameter settings, and adjusting the parameters in the reconstruction for the optimal contrast tailed to each individual patient. Experiments on clinical data have demonstrated the advantages of our approach. Applications in those, such as neonatal brain MRI, where high resolution is critical and contrast is low from direct acquisitions, will be investigated in future work.Acknowledgements
This work was supported in part by the National Institutes of Health (NIH) under Award R01 NS079788, Award R01 EB019483, Award R01 LM013608, Award R01 EB018988, Award R01 NS106030, Award R01 EB031849, Award IDDRC U54 HD090255, Award R01 NS121657, and Award S10OD025111; in part by the Research Grant from the Boston Children’s Hospital Translational Research Program; in part by the Faculty Development Award from Boston Children’s Hospital; in part by the Technological Innovations in Neuroscience Award from the McKnight Foundation; in part by the Research Grant from the Thrasher Research Fund; in part by the Pilot Grant from the National Multiple Sclerosis Society under Award PP-1905-34002; and in part by the Research Grant from American Roentgen Ray Scholarship.References
- Plenge E, Poot DH, Bernsen M, Kotek G, Houston G, Wielopolski P, van der Weerd L, Niessen WJ, Meijering E. Super-resolution methods in MRI: can they improve the trade-off between resolution, signal-to-noise ratio, and acquisition time? Magn Reson Med. 2012, 68(6):1983-93.
- Afacan O, Erem B, Roby DP, Roth N, Roth A, Prabhu SP, Warfield SK. Evaluation of motion and its effect on brain magnetic resonance image quality in children. Pediatr Radiol. 2016, 46(12):1728-1735.
- Sui Y, Afacan O, Jaimes C, Gholipour A, Warfield SK. Gradient-guided isotropic MRI reconstruction from anisotropic acquisitions. IEEE Trans Comput Imaging. 2021, 7:1240-1253.
- Sui Y, Afacan O, Jaimes C, Gholipour A, Warfield SK. Scan-specific generative neural network for MRI super-resolution reconstruction. IEEE Trans Med Imaging. 2022, 41(6):1383-1399.
- Bloch F, Hansen WW, and Packard M. The nuclear induction experiment. Phys Rev. 1946, 70:474–485.
- Van Steenkiste G, Poot DHJ, Jeurissen B, den Dekker AJ, Vanhevel F, Parizel PM, Sijbers J. Super-resolution T1 estimation: Quantitative high resolution T1 mapping from a set of low resolution T1 -weighted images with different slice orientations. Magn Reson Med. 2017, 77(5):1818-1830.
- Barral JK, Gudmundson E, Stikov N, Etezadi-Amoli M, Stoica P, Nishimura DG. A robust methodology for in vivo T1 mapping. Magn Reson Med. 2010, 64(4):1057-67.
- Williams LA, DeVito TJ, Winter JD, Orr TN, Thompson RT, Gelman N. Optimization of 3D MP-RAGE for neonatal brain imaging at 3.0 T. Magn Reson Imaging. 2007, 25(8):1162-70.
- Wang J, He L, Zheng H, Lu ZL. Optimizing the magnetization-prepared rapid gradient-echo (MP-RAGE) sequence. PLoS One. 2014, 9(5):e96899.
- Powell, M J D. An efficient method for finding the minimum of a function of several variables without calculating derivatives. The Computer Journal. 1964, 7: 155-162.
Figures
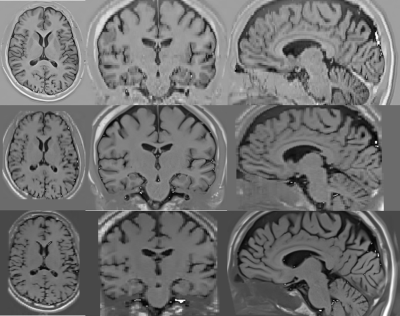
Low-resolution PSIR images acquired from a 3T scanner. The in-plane resolution is 0.8mm x 0.8mm and the through-plane resolution is 2 mm. (top row) axial image with an inversion time of 400 ms; (middle row) coronal image with an inversion time of 800 ms; (bottom row) sagittal image with an inversion time of 1200 ms. All images were acquired with a flip angle of 158 degrees.
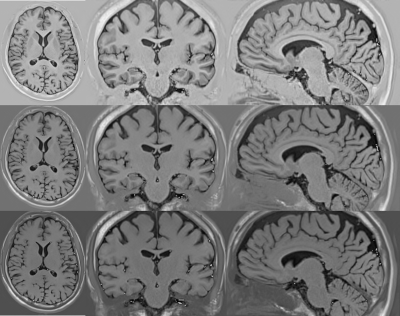
Super-resolved PSIR images at the isotropic resolution of 0.8mm. (top row) axial image with an inversion time of 400 ms; (middle row) coronal image with an inversion time of 800 ms; (bottom row) sagittal image with an inversion time of 1200 ms. The results show that our super-resolution approach offered high-quality images with improved SNR and precise anatomical structures.
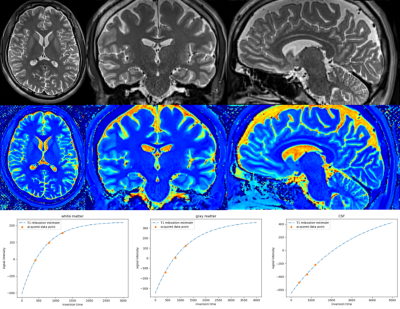
Estimated proton density (top row) and T1 (middle row) mappings from the super-resolved images. (bottom row) Data fitting over the representative voxels from white-matter, gray-matter, and CSF, respectively, according to the T1 relaxation model. The results show that our approach generated accurate and high-resolution proton density and T1 mappings from the super-resolved images.
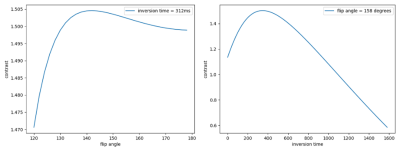
Contrast measures over flip angle (left) and inversion time (right), computed from the estimated proton density and T1 mappings. The results suggest that the contrast measure is eligible to be maximized over inversion time and flip angle.
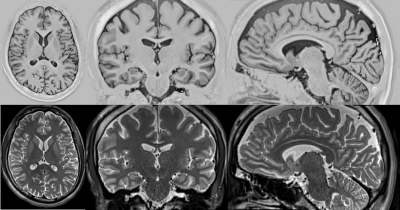
(top row) Reconstructed PSIR image with optimal contrast between gray-matter and white-matter. The optimal contrast was achieved at the inversion time of 312 ms and flip angle of 142 degrees. (bottom row) Reconstructed PSIR image with a T2-like contrast where the inversion time and flip angle were set at 23428 ms and 3.8 degrees, respectively. Note that it is not reasonable to have such a large TI (23428 ms) in practice. This just shows the advantage of our computational approach to reconstruct the image with arbitrary large TI values.
DOI: https://doi.org/10.58530/2023/2206