2178
Abdominal MR Fingerprinting with In-bore Breathing Guidance1Philips Research, Hamburg, Germany, 2Philips GmbH Market DACH, Hamburg, Germany, 3Faculty of Medicine, University of Cologne, Cologne, Germany, 4Institute for Diagnostic and Interventional Radiology, University Hospital Cologne, Cologne, Germany, 5Philips Research, Eindhoven, Netherlands
Synopsis
Keywords: MR Fingerprinting/Synthetic MR, Body, Respiratory motion
MR fingerprinting (MRF) in the abdomen is challenging due to respiratory motion. In this study, as an alternative to a series of extended breath-holds which could be challenging especially for patients, visual and auditive in-bore breathing guidance was used during the MRF scans for better patient comfort. MRF sequences were designed based on predefined breathing patterns such that data are acquired only during exhale holds. The corresponding MRF dictionaries were calculated beforehand to enable real-time matching of quantitative maps. We demonstrate that MRF scans with guided breathing provide comparable abdominal T1 and T2 maps as those with breath-holds.INTRODUCTION
Motion during the MR fingerprinting (MRF) acquisition hinders accurate estimation of quantitative maps because motion alters measured signal evolution whereas it is not represented in the simulated dictionary. Although several methods have been suggested to compensate for motion in MRF, most of them are limited to rigid-body in-plane motion1-3. As the respiratory motion is non-rigid and mostly along through-plane direction for axial acquisitions4, retrospectively correcting motion in abdominal MRF remains challenging. On the other hand, there are approaches that prevent motion-related artifacts prospectively, e.g., breath-holds and respiratory triggering. However, for most cases, a series of long breath-holds is needed to cover a volume of interest, which could be difficult for some patients. Respiratory triggering maximizes patient comfort, but it prevents pre-calculation of the dictionary due to varying data acquisition windows (i.e., exhale durations), making reconstruction time prohibitively long. In this study, to fix the data acquisition window and thus to enable real-time reconstruction while maximizing patient comfort, the breathing guidance system was used where patients are guided to follow a predefined breathing pattern using visual and auditive instructions.METHODS
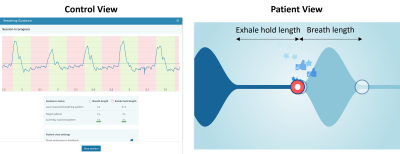
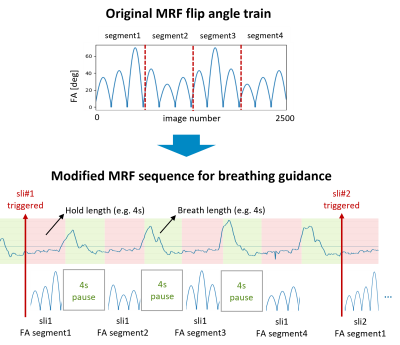
Figure 1 shows an example of control and patient view of the breathing guidance system. The breathing pattern is determined by two parameters, i.e., exhale hold length and breath length. In this study, [exhale hold length – breath length] of [3sec – 3sec] and [4sec – 4sec] were used. The MRF sequence consisting of one inversion pulse (TI=15ms) followed by 2500 RF excitations with varying flip angles5,6 and a constant TR of 6.8ms was modified such that it is synchronized with a target breathing pattern. In other words, a fixed duration of pause (i.e., breath length) was inserted after each data acquisition window of exhale hold length (Figure 2). Each acquisition window started with the minimum flip angle to ensure smooth signal variations. The last acquisition window was filled with RF excitations although the minimum time point of 2500 was reached, resulting in 2567 and 2568 time points for [3sec – 3sec] and [4sec – 4sec] breathing patterns, respectively. The dictionaries corresponding to the MRF sequences with the two breathing patterns as well as the original sequence with 2500 time points for breath-hold were calculated before scanning for real-time reconstruction. T1, T2 and transmit B1 ranges of dictionaries were 50-3000ms, 2-2500ms, and 0.32-1.60, respectively, with multiple step sizes for each range.To demonstrate the feasibility of MRF with breathing guidance, one healthy volunteer was scanned under local ethics approval on a 3.0 T Philips Ingenia scanner (Philips, Best, The Netherlands). Three different FISP-based MRF data were acquired with breath-hold, [3sec – 3sec], and [4sec – 4sec] breathing patterns. The trigger delay was 750ms and 1000ms for the target pattern of [3sec – 3sec] and [4sec – 4sec], respectively. A uniform-density spiral readout with an under-sampling factor of 48 and a readout duration of 2.9ms was used. Other imaging parameters were: field-of-view=440x440mm2, matrix=224x224, slice thickness=5mm, single slice, TE=1.3ms. The total scan duration was 17, 39, and 36 secs for breath-hold, [3sec – 3sec] and [4sec – 4sec], respectively. In addition, a transmit B1 field map was acquired separately using the dual refocusing echo acquisition mode (DREAM)7 technique. For each voxel, entries of the dictionary with the measured transmit B1 value were selected and used for matching.
RESULTS
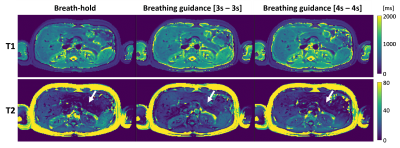
As shown in Figure 3, the MRF scans with breathing guidance provided comparable T1 and T2 maps as breath-hold scans. The T1 maps were robust across all three scans, whereas the T2 maps had unreasonably low values in the pancreas (white arrows) for breath-hold and breathing guidance with [4sec – 4sec].DISCUSSION AND CONCLUSION
The comparable MRF T1 and T2 maps acquired with breath-hold and breathing guidance demonstrated the feasibility of the breathing guidance system for MRF scans. The guided free-breathing scans with the breathing guidance system provide a similar level of patient comfort as compared to respiratory triggering scans while enabling real-time reconstruction of MRF quantitative maps.The scan duration was longer with breathing guidance than breath-hold because data acquisition was paused during breath-in and -out. However, note that for breath-hold scans auditory instructions are played out to guide a subject to follow one extended inhale and exhale cycle before the breath-hold, which adds extra time to the actual MR scan time. In addition, relatively long break times (3-5 respiratory cycles) are usually needed between two consecutive breath-holds. Thus, the total time needed for breath-hold scans may be comparable or even longer than guided breathing scans.
Future works include the optimization of MRF sequences/protocols for more robust MRF T2 mapping.
Acknowledgements
The project received funding from the Federal Ministry of Education and Research of Germany (project number 13GW0364C).References
1. Cruz, G., Jaubert, O., Schneider, T., et al. Rigid motion‐corrected magnetic resonance fingerprinting. Magnetic Resonance in Medicine 2019, 81:947-961.
2. Mehta, B. B., Ma, D., Pierre, E. Y., et al. Image reconstruction algorithm for motion insensitive MR Fingerprinting (MRF): MORF. Magnetic Resonance in Medicine 2018, 2485-2500.
3. Xu, Z., Ye, H., Lyu, M., et al. Rigid motion correction for magnetic resonance fingerprinting with sliding-window reconstruction and image registration. Magnetic Resonance Imaging 2019, 57:303-312.
4. Serrao, E. M., Kessler, D. A., Carmo, B., et al. Magnetic resonance fingerprinting of the pancreas at 1.5 T and 3.0 T. Scientific Reports 2020, 10, 17563.
5. Jiang, Y., Ma, D., Seiberlich, N., et al. MR Fingerprinting Using Fast Imaging with Steady State Precession (FISP) with Spiral Readout. Magnetic Resonance in Medicine 2015, 1621-1631.
6. Chen, Y., Jiang, Y., Pahwa, S., et al. MR Fingerprinting for Rapid Quantitative Abdominal Imaging. Radiology 2016, 279(1):278-286.
7. Nehrke, K. and Börnert, P. DREAM—a novel approach for robust, ultrafast, multislice B1 mapping. Magnetic Resonance in Medicine 2012, 68(5):1517-1526.
Figures