2174
Quantitative assessment of secondary white matter injury in hypothalamic subnuclei by pituitary adenomas utilizing 7-Tesla MRI
Mackenzie Langan1,2, Deborah Marshall3, Bradley Delman4, Raj Shrivastava5, and Priti Balchandani2,6,7
1Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Biomedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Department of Radiation Oncology, Mount Sinai Hospital, New York, NY, United States, 4Diagnostic, Molecular and Interventional Radiology, Mount Sinai Hospital, New York, NY, United States, 5Department of Neurosurgery, Mount Sinai Hospital, New York, NY, United States, 6Diagnostic, Molecular and Interventional Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 7Nash Family Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, United States
1Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Biomedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Department of Radiation Oncology, Mount Sinai Hospital, New York, NY, United States, 4Diagnostic, Molecular and Interventional Radiology, Mount Sinai Hospital, New York, NY, United States, 5Department of Neurosurgery, Mount Sinai Hospital, New York, NY, United States, 6Diagnostic, Molecular and Interventional Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 7Nash Family Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
Keywords: Tumors, Endocrine, Diffusion Tractograpy, hypothalamus
Here we outline a preliminary analysis using a novel method that leverages UHF neuroimaging to measure detectable differences in volumetric, microstructural and tractographic measures in the hypothalamus in patients with pituitary tumors and matched controls. We uncovered significant detectable volumetric and differences within five hypothalamic subnuclei, and the whole left hypothalamus. We observed subtle differences in microstructural integrity, and although not statistically significant warrant further investigations in a larger cohort of pituitary adenoma patients. This may also facilitate exploration of microstructural integrity within other tumor types that may have effects on the hypothalamus.Introduction
The hypothalamus, straddling the inferior margins of the third ventricle, is composed of small subnuclei which contribute to the regulation of numerous autonomic functions and physiological processes such as homeostasis, circadian rhythm stress response, growth, and development(1). The regulation of these functions is well-controlled by the hypothalamic-pituitary-adrenal axis(HPA). Changes within one of these units of this well-regulated feedback loop, as can result from tumors, may result in microstructural or volumetric changes. These changes may have profound effects on hormonal regulation. In patients with pituitary adenomas, changes in the hypothalamus may have profound neuroendocrine effects, result in changes in microstructure or subnuclear volumetric changes(2). Due to its size, proximity to sinuses, inhomogeneity and signal dropout, visualization, and manual segmentation of the whole hypothalamus and individual subnuclei has been difficult to achieve. Compared with conventional field strengths, ultra-high field 7 tesla(UHF)MRI may leverage increased signal-to-noise ratio and improved soft tissue contrast to resolve subtle hypothalamic structural differences in patients with pituitary tumors compared to controls. The combination of UHF MRI and automated deep neural network–based segmentation through FreeSurfer 7.2 enables segmentation of hypothalamic subnuclei with higher accuracy and precision than manual tracing to volumetric changes. These segmentations can then be utilized for high-resolution diffusion tractographic analysis to investigate tissue properties and microstructural integrity. Here, we performed a preliminary analysis using UHF MRI, hypothalamic segmentation and MRtrix in pituitary adenoma patients and matched controls to delineate and segment the hypothalamus in efforts to compare volumetric, microstructural and tractographic metrics from hypothalamic subnuclei.Methods
Ten patients (8 females and 2 males, mean age 45.3 years) with pituitary tumors and were age- and sex-matched with 10 healthy controls (7 females and 3 males, mean age 38.6 years) without current or lifetime history of brain tumors or neurological/psychiatric disorder. Each subject underwent a 7T MRI scan which included a T1-weighted MP2RAGE sequence (TR=6000ms, TE=3.62ms, FOV=240mm×320mm, resolution =0.7mm isotropic) and a high-angular-resolved diffusion-weighted imaging MRI dMRI sequence (b=1500s/mm2,TR=7200ms,TE=67.6ms, FOV=210x210mm, resolution=1.05mm isotropic, 64 directions, and 5 b0 acquisitions). The dMRI series in both anterior-to-posterior and posterior-to-anterior directions were collated into a single volume which was then denoised and corrected for eddy current distortions, motion, and B1 inhomogeneity using MRtrix3. T1 image data was segmented using FreeSurfer7.2 to define the hypothalamus and constituent subnuclei:left/right anterior inferior, anterior superior, posterior, inferior tubular, and superior tubular(Figure 1,Table 1). All images were coregistered into diffusion space. MRtrix3 was used to generate tractography from each nuclei of hypothalamus to whole brain using SIFT2 and tcksample with 1000 seeds per voxel(Figure 2). Statistical analysis was performed using R studio. Age and total intracranial volume were both assessed for normality using Shapiro-Wilks test, and Welch two sample t-test was used to assess if there were significant differences between groups. All measures, Fractional Anisotropy(FA),Mean Diffusivity(MD),streamline count and volume for each subnucleus were assessed for normality. Volumetric differences between groups were assessed using Welch two sample t-test for normal measures, and Mann Whitney U test was used for non-parametric data. FA, MD, and streamline count were assessed using binomial linear regression controlling for age and sex.Results
When assessing differences in volumes, patients had significantly smaller volume of left anterior inferior(p=0.021),left anterior superior(p=0.047),left posterior(p=0.006),right anterior inferior(p=0.005),right anterior superior nuclei(p = 0.020),and whole left hypothalamus(p=0.032)compared to controls(Table 2). There were no significant differences in FA, MD, or count, but there was trending of MD for left posterior(p=0.059)and right anterior superior(p=0.098).Discussion
To our knowledge, this is the first study to acquire diffusion indices of hypothalamic subnuclei within a pituitary adenoma population using advanced spatial resolution afforded by UHF MRI and to assess volumetric, microstructural and tractographic difference in patients and matched controls. Current literature discusses the effects of alterations of the HPA axis and the hypothalamus specifically as it relates to mood and stress response, but the effect that pituitary tumors have on the hypothalamus is still not well understood. Therefore, it is imperative to explore the possible effects on the hypothalamus by exploring possible subtle differences that may contribute to neuroendocrine changes related to volumetric or tractographic changes within specific subnuclei. We found significant reductions in the volume of numerous subnuclei of the hypothalamus including the left and right anterior inferior, which encompass the supraoptic nuclei (SON) that is known to be involved in regulation of vasopressin and oxytocin. We observed subtle microstructural differences in FA, MD, and although not statistically significant, warrant further investigations of hypothalamic microstructural integrity in a larger cohort. These results may provide a basis for exploration of microstructural integrity to establish a deeper understanding of the possible hormonal/neuroendocrine effects associated with changes in specific subnuclei of the hypothalamus in relation to the HPA axis in pituitary adenoma patients. Additionally, results from a larger cohort may contribute towards more informed planning for radiation treatment, may relate to presurgical planning for pituitary adenomas or within other tumor types which may effect the hypothalamusConclusion
We have shown that 7T MRI can be used to segment and delineate the hypothalamus, a key structure of the HPA axis, into individual subnuclei to generate subnuclei specific tractography, in order detect subtle differences within patients with pituitary adenomas compared to healthy controls.Acknowledgements
This work was funded by: NIH R01CA202911References
1. Joseph, D., & Whirledge, S. (2017). Stress and the HPA Axis: Balancing Homeostasis and Fertility. International Journal of Molecular Sciences, 18(10), 2224. https://doi.org/10.3390/ijms18102224
2. Huguet I, Clayton R. Pituitary-Hypothalamic Tumor Syndromes: Adults. [Updated 2015 Jul 3]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278946/
Figures
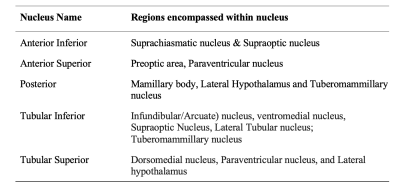
Table one shows the corresponding subnuclei of the hypothalamus as they relate to the generalized segmentation performed through FreeSurfer 7.2.
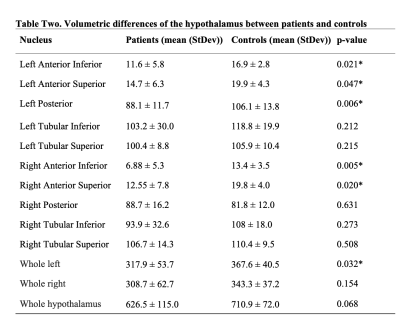
Table two displays the average volumetric differences of each subnuclei for pituitary adenoma patients and matched healthy controls. Significant differences in volume between groups are denoted by *.
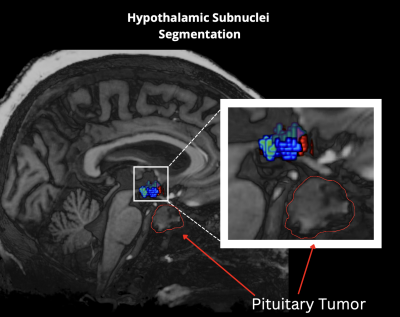
Figure 1. This figure shows the segmentation of the hypothalamic subnuclei. Additionally, it highlights the proximity of the hypothalamus in relation to pituitary tumors which may have effects on surrounding tissue due to their size.
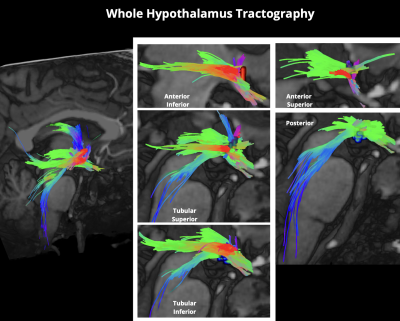
Figure 2. This figure shows the ability to delineate individual hypothalamic subnuclei and generate subnuclei-specific tractography. This enhances our ability to leverage UHF neuroimaging to advance our understanding of how pituitary adenomas may have targeted effects on specific subnuclei which may result in tractographic changes.
DOI: https://doi.org/10.58530/2023/2174