2173
Glymphatic system abnormality in systemic lupus erythematosus identified by diffusion tensor image analysis along the perivascular space1Department of Medical Imaging Center, Nanfang Hospital of Southern Medical University, Guangzhou, China, 2Philips Healthcare, Guangzhou, China
Synopsis
Keywords: Head & Neck/ENT, Brain, Systemic lupus erythematosus
DTI-ALPS is an non-invasive method to measure glymphatic clearance function in brain. Here, we aimed to utilize DTI-ALPS to evaluate the activity of glymphatic system and explore its relationship with grey matter volumes in patients with SLE. Our results demonstrated that the ALPS-index are lower in SLE patients than that in healthy control group. And there were significant correlations between lower ALPS-index and smaller gray matter volumes in some brain regions in SLE group.
Introduction
Up to 80% of patients with systemic lupus erythematosus (SLE) have different degrees of damage in central nervous system (CNS) and cognition functions1, but the clear pathophysiological mechanisms remain unknown. Many studies2,3 have indicated that neuroimmune interfaces dysfunction may occur in SLE. Glymphatic system as a neuroimmune interfaces, its activities have been verified by intrathecally administered tracers in animal and human experiments4-6. Glymphatic system in SLE patients perhaps suffer from similar situation as other neuroimmune interfaces, and a non-invasive method for observing glymphatic function is necessary. Recently, Taoka et.7 proposed a non-invasive method to assess the glymphatic clearance function, which was called the diffusion tensor image analysis along the perivascular space (DTI-ALPS). In this study, we aimed to utilize DTI-ALPS to evaluate the activity of glymphatic system and explore its relationship with grey matter volume (GMV) in patients with SLE.Methods
Forty-six right-handed female patients with SLE (mean age, 29.5 ± 1.4 years) and thirty age- and gender- matched healthy controls (HC) (mean age, 30.5 ± 1.6 years) who underwent conventional MRI, DTI and comprehensive neuropsychological assessment were recruited in this study. All MRI data was acquired on a 3.0 T scanner (Achieva TX; Philips Healthcare, Best, the Netherlands) with an 8-channel head coil for signal reception. 3D-T1WI images subjected to voxel-based morphometry (VBM) analysis were preprocessed using the Statistical Parametric Mapping 12 software package (SPM12) running under MATLAB software. Then the GMV of each brain region were extracted based on the AAL ROI template. The fractional anisotropy (FA) weighted colormap and diffusivity maps in the direction of x-, y-, and z- axes derived from the DTI images were processed on DTI Studio. Four 6-mm-diameter ROIs were placed in bilateral projection fibers and association fibers of the uppermost plane of the lateral ventricle body on the FA-weighted color and diffusivity maps, then the ALPS-index was calculated applying the following formula as Taoka et.7 described: ALPS-index = mean (Dxproj, Dxassoc) / mean (Dyproj, Dzassoc). An example of ROIs placement is shown in Figure 1. The differences in values between SLE patients and HC group were compared using the independent two-samples t-test or Mann–Whitney U test. Correlations between continuous data were determined using Pearson’s correlation analysis. A statistical software package (SPSS 25; IBM, Armonk, NY, United States) was used for analysis, and p values < 0.05 were considered to be statistically significant.Results
There was no significant difference in age and total intracranial volumes between SLE patients and HC group. Table 1 summarizes SLE patients and HC group characteristics and all measurements. SLE patients showed a lower ALPS-index in left (1.539 ± 0.142 vs 1.714 ± 0.175, p < 0.001), right (1.427 ± 0.140 vs 1.556 ± 0.139, p < 0.001) and whole brain (1.483 ± 0.121 vs 1.635 ± 0.139, p < 0.001) compared with the HC group. In all subjects, the ALPS-index of the left side was significantly higher than that of the right side brain (1.608 ± 0.177 vs 1.478 ± 0.153, p < 0.001). VBM analysis exhibited that patients with SLE had smaller GMV in nine brain regions compared with HC group (intensity = 4.843, cluster > 50, FWE p < 0.05). And correlation analysis showed that in SLE group, global GMV was significantly positive correlations with ALPS-index in left-side (p = 0.046), right-side (p = 0.025) and the whole brain (p = 0.013). Left ALPS-index showed a significantly positive correlation with the GMV of bilateral gyrus rectus, left precuneus and left superior temporal gyrus of temporal pole (Figure 2). Right ALPS-index showed a significantly positive correlation with the GMV of right precentral gyrus, left orbital part of middle frontal gyrus, left rolandic operculum, left insula, left precuneus, left superior temporal gyrus and left superior temporal gyrus of temporal pole (Figure 3). Global ALPS-index showed a significantly positive correlation with the GMV of right precentral gyrus, left orbital part of middle frontal gyrus, left rolandic operculum, left gyrus rectus, left insula, left precuneus, left superior temporal gyrus and left superior temporal gyrus of temporal pole (Figure 4).Discussion & Conclusion
Higher ALPS-index represented better glymphatic clearance activity7. Our result showed that the ALPS-index were significantly lower in SLE patients than in HC groups, suggesting the impaired glymphatic drainage. The difference values of ALPS-index between left-side and right-side brain maybe attribute to handedness. Significant correlations were found between lower ALPS-index and smaller gray matter volumes in some brain regions, which are involved in primary motor function8,9 (e.g.: precentral gyrus and rolandic operculum), emotional regulation9-13 (e.g.: orbital part of middle frontal gyrus, rolandic operculum, gyrus rectus, insula, left superior temporal gyrus and left superior temporal gyrus of temporal pole), memory10 (e.g.: orbital part of middle frontal gyrus), auditory processing13 (e.g.: left superior temporal gyrus and left superior temporal gyrus of temporal pole), highly integrated tasks14 (e.g.: precuneus), cognition function10-12 (e.g.: orbital part of middle frontal gyrus, gyrus rectus and insula). Thus, the result may indirectly suggested that atrophy occur in these regions may be related to glymphatic dysfunction in SLE patients. In conclusion, the ALPS-index may be a useful neuroimaging biomarkers for individually quantifying glymphatic activity in patients with SLE.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 81701639).
References
1. Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2:16039. Published 2016 Jun 16.2. Chi JM, Mackay M, Hoang A, et al. Alterations in Blood-Brain Barrier Permeability in Patients with Systemic Lupus Erythematosus. AJNR Am J Neuroradiol. 2019;40(3):470-477.
3. Kamintsky L, Beyea SD, Fisk JD, et al. Blood-brain barrier leakage in systemic lupus erythematosus is associated with gray matter loss and cognitive impairment. Ann Rheum Dis. 2020;79(12):1580-1587.
4. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111.
5. Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123(3):1299-1309.
6. Zhang W, Zhou Y, Wang J, et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage. 2021;238:118257.
7. Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol. 2017;35(4):172-178.
8. Yeo S, Choe IH, van den Noort M, et al. Acupuncture on GB34 activates the precentral gyrus and prefrontal cortex in Parkinson's disease. BMC Complement Altern Med. 2014;14:336. Published 2014 Sep 15.
9. Triarhou LC. Cytoarchitectonics of the Rolandic operculum: morphofunctional ponderings. Brain Struct Funct. 2021;226(4):941-950.
10. Zhao P, Yan R, Wang X, et al. Reduced Resting State Neural Activity in the Right Orbital Part of Middle Frontal Gyrus in Anxious Depression. Front Psychiatry. 2020;10:994. Published 2020 Jan 22.
11. Li W, Lou W, Zhang W, Tong RK, Jin R, Peng W. Gyrus rectus asymmetry predicts trait alexithymia, cognitive empathy, and social function in neurotypical adults [published online ahead of print, 2022 May 15]. Cereb Cortex. 2022;bhac184.
12. Gasquoine PG. Contributions of the insula to cognition and emotion. Neuropsychol Rev. 2014;24(2):77-87.
13. Atmaca M, Koc M, Aslan S, et al. Superior Temporal Gyrus Volumes in Patients With Social Anxiety Disorder. Prim Care Companion CNS Disord. 2021;23(5):20m02815. Published 2021 Aug 26.
14. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564-583.
Figures
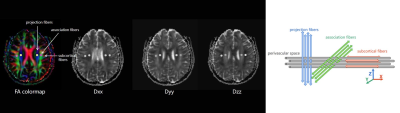
Figure 1: The calculation of index for diffusion tensor image analysis along the perivascular space (ALPS-index). Four 6-mm-diameter ROIs were placed in bilateral projection fibers (blue area) and association fibers (green area) of the uppermost plane of lateral ventricle body on the FA-weighted color and diffusivity maps. The diffusivities in the directions of the x-, y-, and z- axes (Dxx, Dyy, Dzz) of ROIs (grey circle) on projection fibers and association fibers were recorded, respectively. Then the ALPS-index was calculated as [mean (Dxproj, Dxassoc) / mean (Dyproj, Dzassoc)].
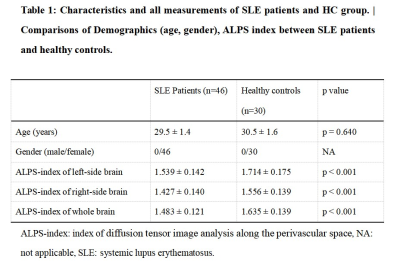
Table 1: Characteristics and all measurements of SLE patients and HC group. | Comparisons of Demographics (age, gender), ALPS-index between SLE patients and healthy controls.
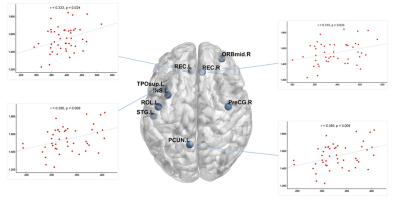
Figure 2: The relationship between left ALPS-index and the GMV of bilateral gyrus rectus, left precuneus and left superior temporal gyrus of temporal pole.
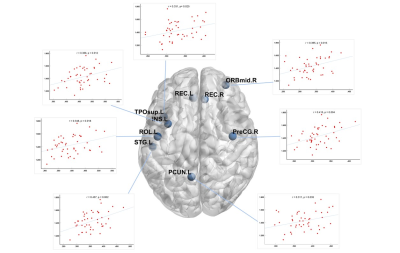
Figure 3: The relationship between right ALPS-index and the GMV of right precentral gyrus, left orbital part of middle frontal gyrus, left rolandic operculum, left insula, left precuneus, left superior temporal gyrus and left superior temporal gyrus of temporal pole.
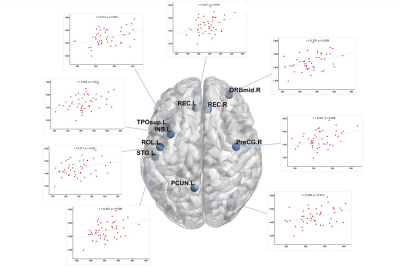
Figure 4: The relationship between global ALPS-index and the GMV of right precentral gyrus, left orbital part of middle frontal gyrus, left rolandic operculum, left gyrus rectus, left insula, left precuneus, left superior temporal gyrus and left superior temporal gyrus of temporal pole.