2167
Efficient fetal brain and placental T1 mapping at 0.55T1Center for the Developing Brain, School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 2Biomedical Engineering Department, School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 4Women's Health, Guy's and St. Thomas' NHS Foundation Trust, London, United Kingdom
Synopsis
Keywords: Quantitative Imaging, Low-Field MRI
Fetal MRI provides crucial anatomical and functional information. At low field it could benefit from reduced distortion, increased bore size and increased B1 homogeneity. T1 contrast and mapping in the fetal brain provides complementary information for example for haemorrhages, infarcts and in placental disease. Here, a low-field (0.55T) fetal T1 inversion-recovery EPI-based sequence benefiting from the lower T1 and hence higher efficiency is demonstrated for placenta and brain in a cohort of pregnant participants including two clinical cases. Significant correlation with gestational age is demonstrated (p<0.05) and high quality maps allowing sub-regions analysis in the brain are shown.Introduction
Fetal development is characterised by a range of fascinating, carefully orchestrated and complex processes. Major pregnancy complications such as pre-eclampsia, fetal growth restriction, fetal anomalies and gestational diabetes are related with deviations in this delicate cascade of events. Their incidence is rising due to an increasingly older and more obese pregnant population. Early identification is key to optimise the outcome for both fetus and mother.While Ultrasound remains the mainstream screening modality, fetal MRI has an important and growing role due, in part to its high-resolution and range of available functional contrasts in addition to anatomical imaging. T1 contrast has been shown to offer complementary information to the more widely used T2-weighted fetal imaging for example for identification of haemorrhage, assessment of the fetal gastro-intestinal system and placental diseases [1]. T1 mapping offers the crucial benefit of quantitative information and, to date, has been mainly employed for the placenta using a range of techniques such as inversion-recovery [2,3,4], Look-Locker sequences [2] and MR Fingerprinting [5], and revealing either no change [2] for Look-Locker [3] or a decrease with increasing gestational age (GA) [2] for inversion-recovery (IR).
Recently re-emerging lower field clinical MRI scanners offer a particular beneficial operating point for fetal imaging due to the reduced field inhomogeneities and thus reduced requirement for specialist correction techniques such as image-based shimming to counter air-tissue interface induced geometric distortion. Furthermore, specifically regarding T1-imaging and mapping, the lower T1 at lower field allows more efficient acquisitions.
This study presents IR-EPI based efficient T1-mapping using slice shuffling as initially presented by Nottingham [5,2] and simultaneous multi-slice on 0.55T in 40 fetal MR examinations, leading to quantitative placental and brain T1 maps.
Methods
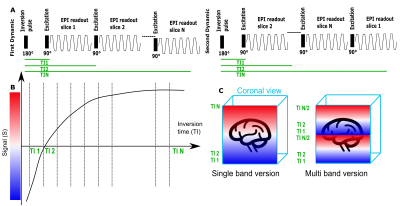
This study was performed on a clinical 0.55T scanner (MAGNETOM Free.Max, Siemens Healthcare, Erlangen, Germany) scanner. A single-shot spin-echo EPI sequence was modified by adding a global (non-selective) inversion pulse, to allow sampling of the recovery of longitudinal magnetization over all Ns slices in the TR. By shuffling the slice acquisition order over Ns subsequent TRs, all slices are sampled at all TIs (see Figure 1A-B). Adding multi-band (MB) acquisition allows the acquisition of two slices simultaneously with the same TI, effectively reducing the number of required dynamics for complete TI sampling by a factor of 2 (Figure 1C). Three protocols were acquired, all sharing a FOV=400x400mm, no in-plane acceleration, no partial-fourier but varying as follows:- I-fast protocol (25-28 slices, Res=4x4x4mm, TE=80ms, TR=3820ms, TIs=[80,..,3725]ms, TA=1:51),
- II-MB (26 slices, Res=4x4x4mm, TE=87ms, TR=2160ms, TIs=[104-2000]ms, TA=1:08, SMS=2)
- III-HR (25-28 slices, Res=3x3x3.1mm, TE=104ms, TR=5000ms, TIs=[104-4874]ms, TA=2:25) - a high-resolution version.
Fetal MRI scans were acquired in a cohort of 40 pregnant women as part of an ethically approved research project (MEERKAT, REC 19/LO/0852, Dulwich Ethics Committee) between May-October 2022 at St Thomas’ hospital, London. Women were scanned in supine position using the 6-channel blanket coil and 9-element posterior coil with continual heart rate and frequent blood-pressure monitoring and verbal interaction. Data was analysed using an in-house built python tool (available in github) resulting in quantitative T1 maps and, after manual segmentation of the placenta and brain, average organ-specific T1 values with gestational trends extracted using linear regression analysis.
Results
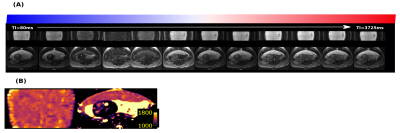
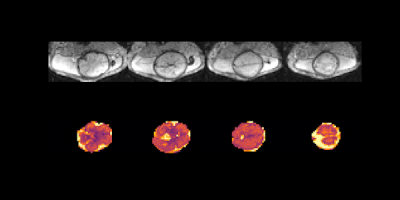
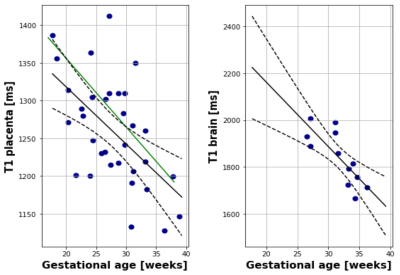
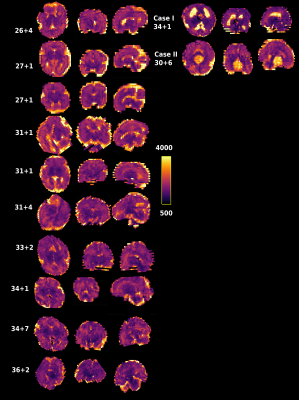
T1 maps were obtained in 40 subjects using protocol I, 16 subjects using protocol II-MB and 11 using III-HR. All were usable for placental T1 maps, 12 were excluded from the brain analysis due to extensive fetal head motion between volumes. Example data over all TIs together with the resulting T1 map is shown in Figure 2 for the placenta as well as animated in Figure 3 for four brain planes. Quantitative evaluation demonstrated significant correlation with gestational age (GA) both for the placenta (Figure 4A, decay of 7.5ms/week, p<0.05), closely aligned to previous literature values at 0.5T (green line) and for the brain (Figure 4B, decay of 27.48 ms/week, p<0.05). Finally, the ability to depict T1 values even for smaller brain regions, thus allowing ROI-analysis in the future, is demonstrated in Figure 5 for seven control brains ordered by GA and in the last row for two clinical cases of bilateral ventriculomegaly and a midline cyst.Dicussion and Conclusions
The use of a contemporary clinical 0.55T scanner allows the seamless integration of own sequence modifications with latest vendor-supplied techniques such as simultaneous multi-slice imaging. Quantitative values were - where literature was available - in-line with previous trends for the placenta. Next steps include motion-correction, region-based T1-analysis for the fetal brain as well as the addition of cohorts diagnosed with pregnancy complications such as pre-eclampsia. Furthermore, the availability of brain-region-specific T1 values will help shape a bespoke standalone T1-weighted acquisition for clinical visualisation and reporting of pathologies in the future.Acknowledgements
The authors thank all pregnant women and their families for taking part in this study. The authors thank the research midwives and radiographers for their crucial involvement in the acquisition of these datasets. This work was supported by a Wellcome Trust Collaboration in Science grant [WT201526/Z/16/Z], a UKRI FL fellowship [MR/T018119/1] and by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z]. The views presented in this study represent these of the authors and not of Guy's and St Thomas' NHS Foundation Trust.References
[1] Aertsen et al, Prenatal Diagnosis. 2020;40:6–17
[2] Gowland PA, Freeman A, Issa B, Boulby P, Duncan KR, Moore RJ, et al. In vivo relaxation time measurements in the human placenta using echo planar imaging at 0.5 T. Magnetic Resonance Imaging 1998 Apr;16(3):241–247
[3] Ingram E, Hawkins L, Morris DM, Myers J, Sibley CP, Johnstone ED, et al. R1 changes in the human placenta at 3 T in response to a maternal oxygen challenge protocol. Placenta 2016;39:151–153.
[4] Ingram E, Morris D, Naish J, Myers J, Johnstone E. MR Imaging Measurements of Altered Placental Oxygenation in Pregnancies Complicated by Fetal Growth Restriction. Radiology 2017;285(3):953–960
[5] Ordidge, R.J., Gibbs, P., Chapman, B., Stehling, M.K. and Mansfield, P. (1990), High-speed multislice T1 mapping using inversion-recovery echo-planar imaging. Magn Reson Med, 16: 238-245.
Figures