2166
Mapping the Accuracy of Diffusion Weighted Imaging Near Total Hip Implants1Hospital for Special Surgery, New York, NY, United States, 2Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Keywords: Quantitative Imaging, Diffusion/other diffusion imaging techniques
Multi-acquisition with variable resonance image combination (MAVRIC) with DWI can reduce artifacts associated with metal implants to characterize synovial reactions near total hip replacements. The purpose of this study was to evaluate the quantitative accuracy of DWI-MAVRIC near actual hip arthroplasty components and demonstrate the feasibility of spatially mapping ADC biases. DWI-EPI and DWI-MAVRIC images were acquired using a diffusion phantom in the presence of femoral heads of different material composition. It was found that cobalt-chromium femoral heads cause more ADC error than oxinium femoral heads. Spatial mapping of ADC accuracy near total hip implants was found to be feasible.Introduction
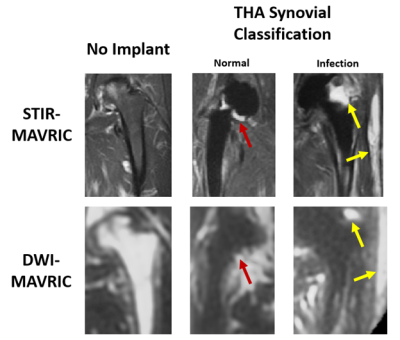
Diffusion weighted imaging (DWI) yields quantitative apparent diffusion coefficient (ADC) measurements which are useful in assessing soft tissues1. Multi-acquisition with variable resonance image combination (MAVRIC) is a novel imaging technique that reduces artifact due to metal implants2. Two-dimensional MAVRIC3 combined with periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER) FSE for DWI4 has been used to characterize synovial reactions near total hip arthroplasties5 (THA)(Figure 1). Previous work evaluating quantitative accuracy of this DWI-MAVRIC acquisition near metal uncovered biases of ADC values, attributable to the multi-shot FSE acquisition6, which would impede interpretation of diffusivity values. Therefore, the goal of this work was to evaluate the quantitative accuracy of DWI-MAVRIC near total hip implant components and demonstrate the feasibility of spatially mapping ADC biases.Methods
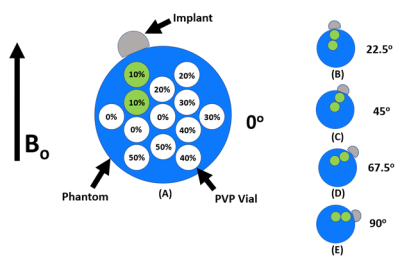
We measured known diffusivity values (DC) of an ADC phantom at different zenith angular (θ:[0o,90o]) and radial (ρ:[ρmin, ρmax]) positions relative to a femoral head (Figure 2) to map the accuracy of the DWI-MAVRIC sequence. Different femoral heads of varying material compositions were placed at a known position and orientation relative to B0.This analysis assumed DC was dependent on susceptibility effects due to B0 and were hence azimuthally-invariant and symmetric about the equator. Extrapolation of DC to (θ:[0o,360o]) and interpolation between unsampled positions were performed by sparse interpolation. Positions that could not be assessed due to signal dropout were excluded.ADC phantom scanning was performed using a Diffusion Phantom Model 128 (CaliberMRI, Boulder, CO). To enable scanning at room temperature and real-time temperature estimation, the phantom was fitted with liquid-crystal vials with MR signal that displays temperature-sensitive MR signal changes7. For this work, two of the 13 vials of polyvinylpyrrolidone (PVP) at 10% wt/wt PVP with diffusivity values closest to soft tissue (1614 µm2/s at 20.5°C) 8 were considered. All images were acquired at iso-center on a clinical 1.5T MRI (MR450, GE Healthcare) with an 8-channel cardiac coil. To assess the effect of orthopedic hardware on ADC accuracy, femoral heads of different sizes and material composition were affixed to the phantom collinear to the two 10% PVP vials (Figure 2). Next, DWI-EPI (NEX= 3) and DWI-MAVRIC (b=600, 3 spectral bins) images were acquired for the following configurations: No metal (NM); 36mm cobalt-chromium (CC) head; 28mm oxidized zirconium head (oxinium [OX]); and 36mm OX. For the configurations with a component present, images were acquired with the phantom rotated 22.5°, 45°, 67.5°, and 90° clockwise in the coronal plane relative to B0 (Figure 2B-E). Internal phantom water temperature was monitored using an SPGR sequence to image liquid crystal vials. Images were processed using in-house software (MATLAB, Mathworks, Natick, MA) to generate ADC maps with gradient nonlinearity correction (GNC)9. ADC values were acquired by placing two circular regions-of-interest (44 voxels, 121 mm2) on each vial using ITK-SNAP10. The actual phantom θ was manually measured from the images, and ρ was measured at 29, 41, 62 and 75 mm from the surface of the implant6. ADC error was calculated using theoretical ADC at the phantom water temperature8 to generate error heat maps of eC=2*(DC – Dtheoretical)/(DC + Dtheoretical), showing error eMetal as a function of (ρ,θ) relative to the implant surface. To offset for effects due to the EPI or PROPELLER pulse sequences, adjusted error (eMetal–eNM) heat maps were also generated, using in-house software (MATLAB, Mathworks, Natick, MA).
Results
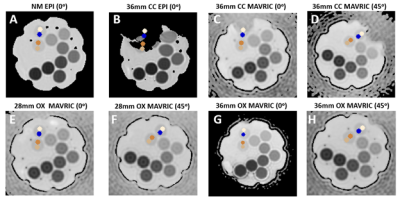
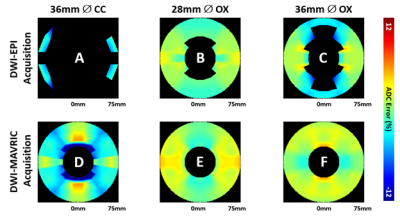
Figure 3 shows ADC maps in all four scanning configurations, displaying severe image distortion with the EPI acquisition and CC femoral head. DWI-MAVRIC shows its largest bias patterns at θ=0o and with CC. The DWI-EPI eNM bias was small and negative (~ 2%), while DWI-MAVRIC eNM showed a strong positive bias(~3-6%). Offsetting by eNM , the greatest errors eCC–eNM occurred at the angles of 0o and 180o for both DWI-EPI and DWI-MAVRIC, followed by 90o and 270o for both DWI-EPI and DWI-MAVRIC (Figure 4). Overall, DWI-MAVRIC acquisitions showed less error as compared to DWI-EPI. CC implants caused higher eMetal–eNM spatial variation than OX. The 28 mm OX showed less error than 36 mm OX for DWI-EPI, but produced a different error pattern in DWI-MAVRIC.Discussion
This study demonstrated the feasibility for mapping diffusivity bias patterns using the DWI-MAVRIC sequence in the presence of different femoral head components commonly used in total hip arthroplasty. We observed a positive DWI-MAVRIC bias similar to previous work6,11 with bilobular bloom patterns that conform to well-known B0 susceptibility patterns6. These results suggest that distance from the metal implant, zenith angle relative to the metal, and material composition of the implant should be considered when evaluating ADC values in close proximity to implanted metallic devices. In this preliminary demonstration, we limited the measurements to only five zenith angles and four distances, which may be increased to improve data interpolation of the heat maps. ADC data also could not be acquired near the surface of the implant which prevents conclusions from being drawn about ADC accuracy immediately adjacent to implanted hardware.Conclusion
DWI-MAVRIC has less ADC error than DWI-EPI when imaging in the presence of hip implants. Cobalt-chromium femoral heads cause more ADC error compared to oxinium femoral heads. Spatial mapping of ADC accuracy near total hip implants was found to be feasible.Acknowledgements
The authors would like to thank the MRI administrative staff and MRI technologists at Hospital for Special Surgery for their assistance in acquiring images. Research reported in this publication was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R01AR064840 and National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health R21EB023415 and R21EB030123. This content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.References
1.Bihan D le. Apparent diffusion coefficient and beyond : What diffusion mr imaging can tell us about tissue structure. Radiology. 2013;268(2):318-322. doi:10.1148/radiol.13130420
2. Koch KM, Lorbiecki JE, Hinks RS, King KF. A multispectral three-dimensional acquisition technique for imaging near metal implants. Magn Reson Med. 2009;61(2):381-390. doi:10.1002/mrm.21856
3. Hargreaves BA, Taviani V, Litwiller D v, Yoon D. 2D multi-spectral imaging for fast MRI near metal. Magn Reson Med. 2018;79(2):968-973. doi:https://doi.org/10.1002/mrm.26724
4. Koch KM, Bhave S, Gaddipati A, et al. Multispectral diffusion-weighted imaging near metal implants. Magn Reson Med. 2018;79(2):987-993. doi:10.1002/mrm.26737
5. Gao MA, Neri JP, Lin B, et al. Diffusion MRI of THAs for the Classification of Synovial Reactions. In: ISMRM. 2021.
6. Neri J, Koff M, Koch K, Tan E. Quantitative Assessment of Diffusion Weighted Imaging Near Metal Implants. In: ISMRM. 2022.
7. Keenan KE, Stupic KF, Russek SE, Mirowski E. MRI-visible liquid crystal thermometer. Magn Reson Med. 2020;84(3):1552-1563. doi:10.1002/mrm.28224
8. Wagner F, Laun FB, Kuder TA, et al. Temperature and concentration calibration of aqueous polyvinylpyrrolidone (PVP) solutions for isotropic diffusion MRI phantoms. PLoS One. 2017;12(6):1-13. doi:10.1371/journal.pone.0179276
9. Tan ET, Marinelli L, Slavens ZW, King KF, Hardy CJ. Improved correction for gradient nonlinearity effects in diffusion-weighted imaging. J Magn Reson Imaging. 2013;38(2):448-453.doi:10.1002/jmri.23942
10. Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116-1128. doi:10.1016/j.neuroimage.2006.01.015
11. Neri JP, Koff MF, Koch KM, Tan ET. Validating the accuracy of multispectral metal artifact suppressed diffusion-weighted imaging. Med Phys. Published online October 1, 2022. doi:10.1002/mp.15925
Figures