2163
Parallel transmit (pTx) kT-points pulses improve 500µm resolution quantitative multi-parameter mapping (MPM) at 7T1Department of Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Spinal Cord Injury Center Balgrist, University Hospital Zurich, University of Zurich, Zurich, Switzerland, 4Felix Bloch Institute for Solid State Physics, Faculty of Physics and Earth Sciences, Leipzig University, Leipzig, Germany
Synopsis
Keywords: Quantitative Imaging, Parallel Transmit & Multiband, Neuro
Quantitative multi-parameter mapping (MPM) at 7T can provide detailed information about brain microstructure. However, it is limited by B1+ radio-frequency (RF) transmit field inhomogeneities leading to bias and dropouts. We implemented a kt-points RF pulse approach in 500µm resolution multi-echo spoiled GRE acquisitions and AFI B1+ mapping used for MPM. It was optimized for a homogeneous non-selective excitation with short RF pulse durations, SAR compliance and high usability. In a group of 8 volunteers, bias and shading artifacts were significantly reduced. The approach is integrated in the routine scanning workflow, improving whole brain ultra-high resolution MPM at 7T.Introduction
Quantitative multi-parameter mapping (MPM) can provide detailed information about brain microstructure at 7T1,2, measuring: longitudinal relaxation rate (R1), proton density (PD) and effective transverse relaxation rate R2*. It is based on a combination of 3D multi-echo gradient echo (GRE) acquisitions, B1+ radio-frequency (RF) transmit field mapping and modeling2,3. Despite including B1+ mapping in the quantification, bias and shading artifacts were observed in some basal and central brain areas, primarily due to severe RF transmit field inhomogeneities giving suboptimal local excitation flip angles.The combination of parallel transmit (pTx) and special pulse design can significantly reduce RF excitation inhomogeneities4. We integrated the pTx kt-points approach for non-selective excitation with high homogeneity in the GRE and AFI5 acquisitions. To maintain the high acquisition speed, resolution and routine use of MPM at 7T, we aimed at short RF pulse durations, compatibility with ultra-high resolution, SAR compliance and seamless integration in the standard scanning workflow. The performance of the kt-points MPM approach was compared to the conventional MPM approach in a group of 8 volunteers.
Methods
Eight volunteers were scanned with the conventional and kt-points MPM approach on a 7T MAGNETOM Terra scanner (Siemens Healthcare, Erlangen, Germany) using the standard 8-channel transmit and 32-channel receive RF head coil (Nova Medical, Wilmington, USA).The pTx kt-points excitation was integrated into the MPM approach established for ultra-high resolution quantitative imaging2. Data were acquired with two different non-selective RF excitation pulses: pTx 4 kt-points (220µs sub-pulses) and sinc pulses (180, 1368µs pulse durations, BWT 6), here called pTx and True Form acquisition, respectively. The MPM acquisition entailed two in-house built 3D multi-echo GRE with PD- and T1-weighting (with nominal excitation flip angles of 8° and 24° respectively for the True Form measurement, and 7° and 22° for the pTx measurement) with: 500µm isotropic resolution, matrix size 434 x 496 x 352, TR 23.5ms, six evenly spaced bipolar gradient readout echoes (TE 3.22 - 16.0ms), 2x2 GRAPPA acceleration, gradient and RF spoiling applied, TA 17:20 min per weighting. Maps of the local flip angle distribution were estimated from an in-house built AFI5 (4mm isotropic resolution, flip angle of 55°, TR1/TR2 25/125ms, TA 3:52 min), which used the same shape of RF excitation pulse to ensure direct comparability and applicability of RF transmit maps.
The kt-points pulse was optimized to achieve a homogeneous 22° excitation (at 1.3ms maximal duration and within SAR limits) for each individual across the entire brain. A magnitude least-squares optimization of magnitude and phase of the RF pulse used B0 and B1+ maps acquired during the adjustment procedure (total time ~2.5 min). The pulse amplitudes were proportionally scaled to achieve the 7° and 55° flip angles for PD-weighting and AFI acquisitions.
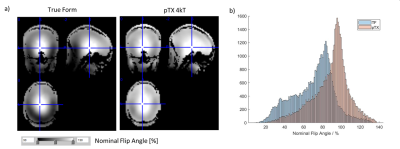
Data were processed using the open source hMRI toolbox (hMRI.info3) with 7T processing parameters2. It resulted in local flip angle maps (in percent of nominal flip angle), R1, PD and R2* maps. Maps were transformed to MNI group space for further analysis using DARTEL as implemented in the hMRI toolbox. The voxel-wise mean, standard deviation (SD) and coefficient of variation (CoV) were calculated across the group for each method to compare noise and bias characteristics.
Results
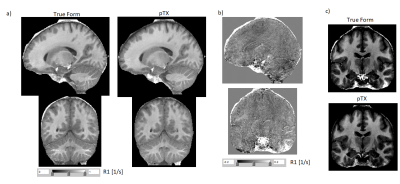
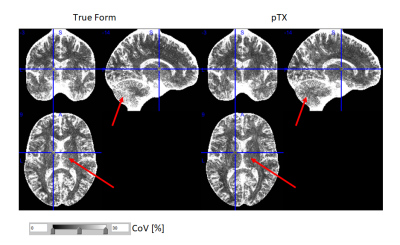
The pTx approach significantly improved the excitation flip angle homogeneity across the brain (Fig.1). This improvement translated to lower bias (Fig.2) and reduced CoV (Fig.3) in R1 maps. The pTx approach also improved CoV of the PD maps but not for R2* maps (Fig.4).Discussion
The pTx approach most prominently improved homogeneity and CoV of R1 maps and to a lesser extent PD maps, but had little impact on the R2* maps. Improvements were most conspicuous in areas typically suffering from signal dropouts due to low effective flip angles such as the inferior temporal lobes or parts of the cerebellum. Interestingly, reduced CoV in R1 maps was also observed in the center of the brain, since the RF dome effect was reduced and thus conditioned the R1 estimation better. The negligible impact on R2* maps can be explained by the small dependence of R2* fits on the initial signal intensity.Residual artifacts in the maps may be due to other factors such as head motion, physiology or inadvertent magnetization transfer (MT) effects, which all may be further reduced2,6. However, the MT effects were generally lower than for conventional True Form excitation, since the nominal flip angle (and thus power) could be lowered while achieving sufficient excitation in basal brain areas.
Since the same kt-points pulse is used for the multi-echo GRE acquisitions and AFI B1+ mapping, the data can be processed in the same way as conventional MPM data without the need to process the B1+ maps further.
Conclusion
The pTx kt-points approach significantly improved homogeneity and CoV in R1 maps, PD maps and impacted R2* maps to a much smaller degree. The improvements in a group of 8 volunteers indicate a larger effective coverage and sensitivity for anatomical studies. The approach can be readily deployed and integrated in the scanning and data processing workflow as it only adds ca. 2.5 mins scan time, runs on the scanner console and is compatible with established quantitative MRI processing pipelines (e.g., hMRI toolbox).Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement n° 616905; from the European Union's Horizon 2020 research and innovation programme under the grant agreement No 681094; from the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 15.0137; from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – project no. 347592254 (WE 5046/4-2); from the Federal Ministry of Education and Research (BMBF) under support code 01ED2210.References
[1] Weiskopf N, Edwards LJ, Helms G et al. Quantitative magnetic resonance imaging of brain anatomy and in vivo histology. Nat. Rev. Phys. 2021; 3(8):570-588.
[2] Vaculčiaková L, Podranski K, Edwards LJ et al. Combining navigator and optical prospective motion correction for high-quality 500 μm resolution quantitative multi-parameter mapping at 7T. Magn Reson Med. 2022; 88(2):787-801.
[3] Tabelow K, Balteau E, Ashburner J et al. hMRI – A toolbox for quantitative MRI in neuroscience and clinical research. Neuroimage. 2019; 194:191-210.
[4] Cloos MA, Boulant N, Luong M et al. kT -points: short three-dimensional tailored RF pulses for flip-angle homogenization over an extended volume. Magn Reson Med. 2012; 67(1):72-80.
[5] Lutti A, Stadler J, Josephs O et al. Robust and fast whole brain mapping of the RF transmit field B1 at 7T. PLoS One. 2012; 7(3):e32379.
[6] Leitão D, Tomi-Tricot R, Bridgen P et al. Parallel transmit pulse design for saturation homogeneity (PUSH) for magnetization transfer imaging at 7T. Magn Reson Med. 2022; 88(1):180-194.
Figures